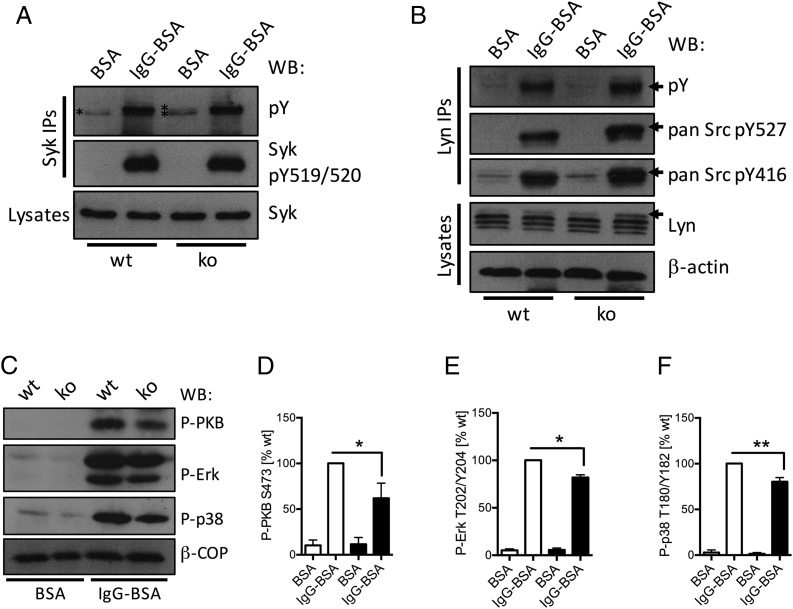
FIGURE 5.
Neutrophil PTPN22 regulates Lyn and Syk tyrosine phosphorylation, which affects the activity status of many signaling cascades. Neutrophils isolated from bone marrows of Ptpn22−/− [knockout (ko)] and matched WT control mice were allowed to adhere to immobilized ICs (IgG-BSA) or, as a control, BSA for 12 min. (A and B) Lysates were prepared as detailed in Materials and Methods for Western blotting, using the indicated Abs; in addition, Syk (A) or Lyn (B) was immunoprecipitated for analysis of phosphotyrosine as well as probing with Abs detecting specific tyrosine phosphorylations in Syk/SFK proteins as indicated. Representative samples from at least three separately performed experiments are shown. (C–F) Lysates were prepared and subjected to immunoblotting with Abs specific for phospho-PKB, phospho-Erk, and phospho-p38 MAPK (C–F). Blots were quantitated using Image J software. (C) Representative blots. (D–F) Densitometry from five separate experiments (means ± SEM) was normalized to activated controls. *p < 0.05, **p < 0.01.