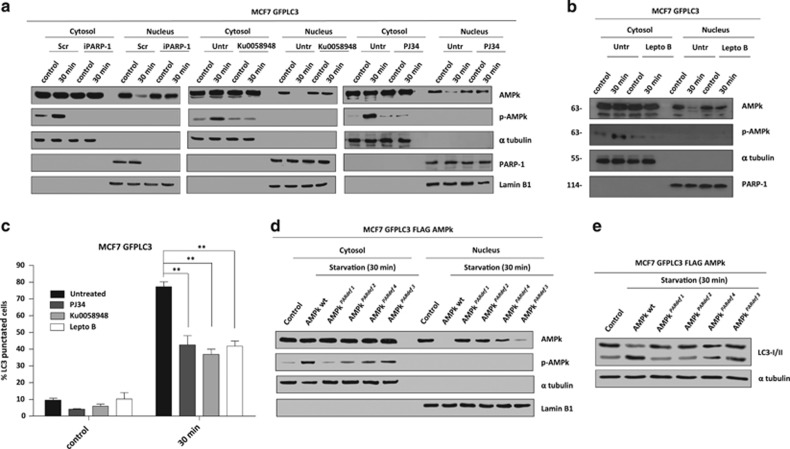
Figure 5.
PARylation and nuclear export of AMPk. (a) Nuclear export of AMPk during starvation. The nuclear and cytosolic fractions were prepared from fed or starved MCF7-GFPLC3 cells, co-treated or not with PJ34 (10 μM) or KU0058948 (100 nM) or silenced for PARP-1. PJ34 and KU0058948 were used as pre-treatment during 2 h and maintained during starvation assay. (b) Leptomycin B prevents nuclear export of AMPkα1 during starvation. MCF7-GFPLC3 were pre-treated with Leptomycin B 20 ng/ml during 3 h and maintained during starvation with HANK buffer for 30 min. (c) Effect of Leptomycin B, PJ34 and KU0058948 on autophagy levels. MCF7 GFPLC3 cells were co-treated with 20 ng/ml Leptomycin B, 10 μM PJ34 or 100 nM KU0058948 and starved with HANK Buffer 30 min. Cells with the typical punctated pattern of starvation-induced LC3 was counted by fluorescence microscopy. Similar results were obtained in three independent experiments. At least 250 cells were counted in a Zeiss fluorescent microscope in 3 independent experiments. **P<0.01 versus the indicated group by t-test. (d) Nuclear export of AMPk during starvation is blocked by PARylation sites mutation in AMPkα1. MCF7 GFPLC3 cells were transfected with FLAG-AMPkα1 wildtype and AMPKPARdef mutants. (e) Mutation in PARylated sites of AMPkα1 blocks autophagy induction. MCF7 cells transfected with FLAG-AMPkα1 wildtype and mutants (PARdef 1-4), and starved for 30 min. Autophagy induction was assessed by specific endogenous LC3-II translocation. Similar results were obtained in three independent experiments. In A, B and D figures, total levels and activation of AMPk (Phospho-AMPk) were analyzed in both fractions. α-tubulin and PARP-1 and/or Lamin B1 were used as cytosolic and nuclear fractions respectively. Similar results were obtained in three independent experiments