Figure 2.
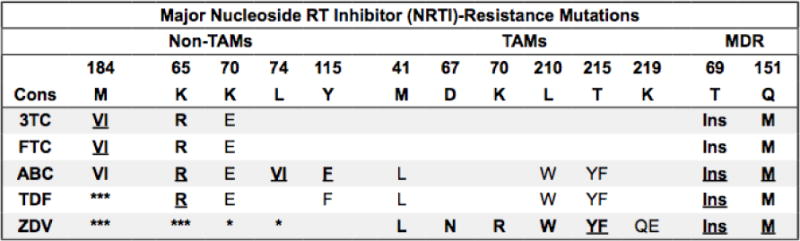
Summary of nucleoside/nucleotide reverse transcriptase inhibitor drug resistance mutations
Bold/underline: High-level reduced susceptibility or virological response. Bold: reduced suceptibility or virological response. Plain text: reduced susceptibility in combination with other NRTI-resistance mutations. Asterisk: increased susceptibility. Additional NRTIs: Stavudine (d4T) and didanosine (ddI) are no longer recommended. TAMs: Thymidine analog mutations. Selected by AZT and d4T and facilitate primer unblocking. Non-TAMs prevent NRTI incorporation. MDR: Multidrug resistance mutations. T69 insertions occur with TAMs. Q151M occurs with non-TAMs and accessory mutations A62V, V75I, F77L, and F116Y. M184VI: Although they cause high-level in vitro resistance to 3TC/FTC, they are not contraindications to 3TC/FTC because they increase TDF and AZT susceptibility and decrease viral replication fitness. Additional mutations: K65N is similar but weaker than K65R. K70GQ is similar to K70E. T69D and V75MT reduce susceptibility to d4T and ddI. T215SCDEIV (T215 revertants) evolve from T215YF in the absence of NRTIs. E40F, E44DA, D67GE, V118I, and K219NR are accessory TAMs. T69 deletions occur in combination with K65R and/or Q151M. With K65R (but not Q151M) they increase AZT susceptibility. References: http://hivdb.stanford.edu/DR/NRTIResiNote.html.
