Figure 4.
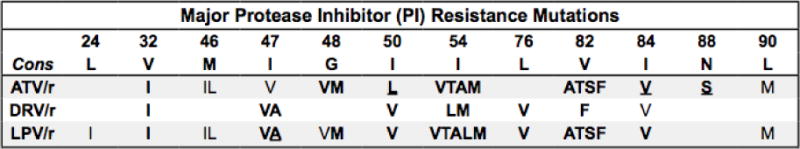
Summary of protease inhibitor drug resistance mutations.
Bold/underline: High-level reduced susceptibility or virological response. Bold: reduced suceptibility or virological response. Plain text: reduced susceptibility in combination with other PI-resistance mutations. Abbreviations: atazanavir (ATV), darunavir (DRV), lopinavir (LPV). Administered with ritonavir for pharmacokinetic boosting (/r). Additional PIs: Fosamprenavir (FPV), indinavir (IDV), saquinavir (SQV), and tipranavir (TPV) are rarely used. Nelfinavir (NFV) is no longer recommended. FPV/r and IDV/r are never more active than DRV/r and rarely if ever more active than LPV/r vs resistant viruses. TPV/r is occasionally useful for salvage therapy as it can be active vs LPV/r and DRV/r-resistant viruses with mutations that increase TPV susceptibility. Expert consultation +/− phenotypic testing should be obtained prior to using FPV, FPV/r, IDV/r, SQV/r, and TPV/r. Additional mutations: D30N and N88D are major NFV-resistance mutations. L10F, V11I, K20TV, L23I, K43T, F53L, Q58E, A71IL, G73STCA, T74P, N83D, and L89V are common nonpolymorphic accessory mutations. L10RY, V11L, L24F, M46V, G48ASTLQ, F53Y, I54S, V82CM, I84AC, N88TG are rare nonpolymorphic variants. Hypersusceptibility: I50L (each PI except ATV); L10F, L24I, I50V, I54L (TPV); L76V (ATV, SQV, TPV); I47A (SQV); N88S (FPV). References: http://hivdb.stanford.edu/DR/PIResiNote.html.
