Abstract
Hypobaric hypoxia at high elevation represents an important physiological stressor for montane organisms, but optimal physiological strategies to cope with hypoxia may vary among species with different life histories. Montane birds exhibit a range of migration patterns; elevational migrants breed at high elevations but winter at low elevations or migrate further south, while high-elevation residents inhabit the same elevation throughout the year. Optimal physiological strategies to cope with hypoxia might therefore differ between species that exhibit these two migratory patterns, because they differ in the amount time spent at high elevation. We examined physiological parameters associated with blood-oxygen transport (haemoglobin concentration and haematocrit, i.e. the proportion of red blood cells in blood) in nine species of elevational migrants and six species of high-elevation residents that were sampled along a 2200 m (1000–3200 m) elevational gradient. Haemoglobin concentration increased with elevation within species regardless of migratory strategy, but it was only significantly correlated with haematocrit in elevational migrants. Surprisingly, haemoglobin concentration was not correlated with haematocrit in high-elevation residents, and these species exhibited higher mean cellular haemoglobin concentration than elevational migrants. Thus, alternative physiological strategies to regulate haemoglobin concentration and blood O2 carrying capacity appear to differ among birds with different annual elevational movement patterns.
Keywords: haemoglobin concentration, haematocrit, elevational migration, birds, comparative physiology, Himalayas
1. Introduction
A primary goal of comparative physiology is to determine whether ecological similarities among species might explain similarities in their adaptive responses to common selective pressures [1]. Matching migratory strategies, for instance, can influence the temporal and spatial variation in environmental stressors individuals of a species experience over their lifetime. As a result, species that share key life-history traits may exhibit analogous physiological strategies to cope with the same environmental stressor [2]. Species that are distributed along elevational gradients are powerful systems to investigate the influence of life-history variation on physiological adaptation. Key abiotic stressors such as temperature and biotic stressors such as interspecific competition [3] change with elevation. Certain abiotic stressors intensify predictably with elevation (e.g. the decrease in temperature with increase in elevation), and dramatic changes in these stressors occur over relatively small spatial scales. Perhaps most notable is the decrease in the partial pressure of oxygen (hypobaric hypoxia) at high elevations [4]. Hypobaric hypoxia is constant and predictable at a given elevation around the world throughout the year and is not affected by latitude or season, making it a unique stressor for high-elevation organisms globally. Organisms cope with hypoxia using diverse physiological and behavioural strategies [1], but systematic analyses of the influence of life-history characteristics on the utilization of these alternative strategies are rare.
Studies of physiological responses to hypoxia in laboratory animals (e.g. guinea pigs and rats) [5,6], humans [7,8] and free-living wild species [9–11] have revealed a diverse suite of physiological modifications that species can use to counter hypoxia. Among the most common are increases in pulmonary O2 transport [12] and modification of blood O2 carrying capacity [13]. An important determinant of blood O2 carrying capacity is haemoglobin concentration (henceforth [Hb]) [14,15]. Erythropoietic responses to hypobaric hypoxia can increase blood O2 carrying capacity by increasing [Hb] via the proliferation of red blood cells, which leads to an increase in haematocrit (henceforth Hct), the proportion of whole blood volume that is occupied by erythrocytes. This erythropoietic response to hypoxia is well documented in birds [16] and other vertebrates that routinely migrate between highland and lowland environments [17]. Although this response allows of fine-tuning of blood O2 carrying capacity as a potentially effective short-term solution to hypoxic challenge, the adaptive value of chronically elevated Hct in high-elevation organisms is questionable.
Although increasing [Hb] through increases in Hct can increase blood O2 carrying capacity, elevated Hct also increases blood viscosity, and excessive blood viscosity can increase cardiac load and hamper effective blood circulation, both of which may ultimately decrease convective oxygen delivery. Given these considerations, several studies have suggested that Hct and [Hb] values that optimize aerobic output in humans are very close to those typically observed at sea level [12,13,15,16]. Not only does chronically elevated Hct potentially reduce aerobic output, it is associated with pathophysiological conditions in humans (e.g. chronic mountain disease), and, correspondingly, many high-elevation specialist species have evolved blunted erythropoietic responses to hypoxia [17] (normal Hct at high elevations). Thus, the optimal erythropoietic response to hypoxia may vary according to the amount of time an organism spends under hypoxic conditions.
Birds in the Himalayas exhibit two distinct phenologies of elevational distribution. Elevational migrants occur at low elevation for most of the year (eight to nine months), but move to higher elevations (2000–4000 m or even higher) during the three- to four-month summer to breed [18]. These two elevational movement strategies are widely known in birds and have been hypothesized to arise due to limits on cold tolerance in winter [19], food availability [20] and predation [21]. These differences result in elevational migrants enduring significant hypoxic stress only for a short period of the year compared with high-elevational residents, which occur at high elevation throughout the year. Given their contrasting elevational movement patterns, elevational migrants and resident birds present an ideal system with which to understand if elevational movement patterns explain the strategies to cope with environmental hypoxia through erythropoietic regulation of blood O2 carrying capacity.
In this study, we used a survey of 15 avian species that differ in migratory strategies to demonstrate that erythropoietic responses to increasing elevation can be explained by interspecific differences in annual elevational movement patterns. We predicted elevational migrants would show a seasonal increase in [Hb] with a correlated increase in Hct during the breeding season because the ability to finely tune blood O2 carrying capacity may outweigh the maladaptive but transient increases in blood viscosity. High-elevation residents, on the other hand, experience chronic hypoxia, and may thus exhibit alternative strategies for coping with hypobaric hypoxia, which may include a blunted erythropoietic response to increasing elevation as has been documented in other high-elevation specialists. Using this comparative approach, we demonstrate that differences in species' annual elevational movement patterns (elevational migrants and high-elevation residents) can explain differences in their physiological responses to abiotic stressors. To the best of our knowledge, this is the first study to link interspecific variation in migratory strategies to variation in erythropoietic responses to hypoxia.
2. Material and methods
(a). Field methods
We obtained blood samples from 15 species of passerine birds (figure 1) that we caught opportunistically using mist-nets at seven elevations: 1000, 1500, 2100, 2650, 2800, 3000 and 3200 m in the Amrut Ganga Valley of Kedarnath Wildlife Division, Uttarakhand, India, These species were selected based on their abundance and diversity in elevational distribution. We restricted our analyses to passerine species to minimize the effect of evolutionary distance on differences in physiology. We sampled birds in two seasons—summer/breeding season (15 March–30 June) and winter/non-breeding season (1 January–15 March)—in 2014 and 2015. We used 15 March across both years of sampling as the date for the onset of the breeding season (summer) as resident birds were commonly seen singing about a week before this date [18]. To ensure that we did not sample elevational migrants that had just arrived on their breeding grounds and not acclimatized to the hypoxia, we sampled at and above 2600 m only in late April and May. As our study sites spanned an elevational gradient larger than the distribution of most species, at each sampling location, only a subset of the species sampled was present (figure 1). For each blood sample, we measured whole blood [Hb] with a Hemocue 201+ analyser using the manufacturer's protocol (HemoCue AB, Ängelholm, Sweden; nbreeding season = 356). Whenever sufficient sample was available, we also measured Hct for the same samples, using a Zipocrit Portable Centrifuge (LW Scientific Inc., Lawrenceville, GA, USA) with a spin time of 5 min (nbreeding season = 178, nnon-breeding season = 152). We calculated the Mean Cellular Haemoglobin Concentration (henceforth MCHC) using the formula MCHC = ([Hb] × 100/Hct) (nbreeding season = 178, nnon-breeding season = 152) [24]. We checked for repeatability of our measures by taking two successive measurements from the same bird for [Hb] (n = 21 birds) and Hct (n = 23 birds), and calculating the intraclass correlation coefficient (ICC) using the package ICC in the R software package v. 3.2.1 [25]. For both measures, we had high ICC scores ([Hb] = 0.85, Hct = 0.98), confirming our measurements were highly repeatable.
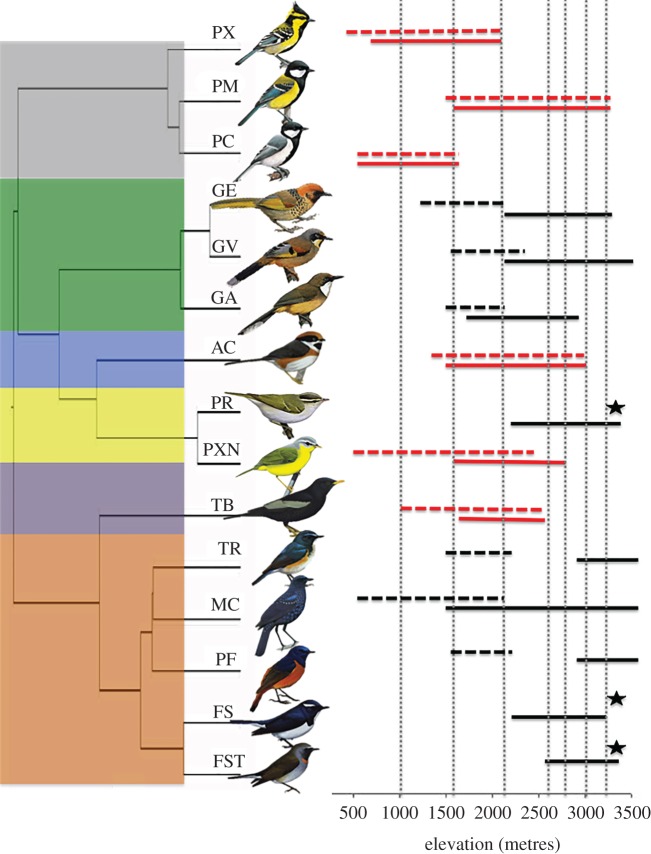
Figure 1.
Evolutionary relationships among species used in the analysis with phylogeny recreated using a posterior set of pruned trees from Jetz et al. [22]. Elevational distributions are from Dixit et al. [18]. Dotted vertical lines represent sampling locations. Horizontal solid lines represent species breeding elevational distribution and dashed lines represent species winter distribution (in metres) of resident (red) and EM (black) species. Stars indicate species with winter distribution outside the study area. Coloured boxes on the left margin denote taxonomic families used in the analysis: grey, Paridae; green, Timalidae; blue, Aegithalidae; yellow, Sylvidae; purple, Turdidae; buff, Muscicapidae. Species codes (from top): PX (Parus xanthogenys), PM (Parus monticolus), PC (Parus cinereus), GE (Garrulax erythrocephalus), GV (Garrulax variegatum), GA (Garrulax albogularis), AC (Aegithalos concinnus), PR (Phylloscopus reguloides), PXN (Phylloscopus xanthoschistos), TB (Turdus boulboul), TR (Tarsiger rufilatus), MC (Myophonus caeruleus), PF (Phoenicurus frontalis), FS (Ficedula superciliaris), FST (Ficedula strophiata). Bird illustrations were reproduced from Handbook of the Birds of the World Alive [23].
(b). Data analyses
Based on information from field surveys [18] and information from the literature [26], we determined elevational distribution (in both winter and summer) of each species and mass (g) (electronic supplementary material, S1). Most birds in the Himalayas show some elevational movement, especially during extreme weather events. We classified birds as elevational migrants (EM status = 1) for species where almost all individuals migrate to lower elevations from the upper 75% of their breeding range. Sedentary residents (EM status = 0) were species that are consistently found in 75% of their breeding range in the winter in our study site.
To understand the variation in the relationship between [Hb] and Hct among species that exhibit the two alternative elevational movement patterns, we quantified the strength of the relationship between [Hb] and Hct separately using linear regression in elevational migrants (nine species) and residents (six species). To further understand the mode of increase in [Hb] in individuals showing elevated [Hb], we compared the MCHC in the upper quartile values of [Hb] between elevational migrants and residents. Finally, using data for 15 species in summer and nine species in winter, we fitted 10 linear mixed models and used AIC-based multi-model inference to identify well-supported statistical models that describe the relationships between [Hb] and biological parameters relevant to elevational distribution, such as (i) Hct, (ii) elevational range, (iii) range upper limit (highest limit of distribution for the species in the landscape), (iv) range position calculated as (elevation where bird was caught − lower elevational limit of distribution)/elevational range of species, (v) EM status (1 = elevational migrants, 0 = resident species) and (vi) mass (g) as fixed effects. In these models, we controlled for phylogenetic effects by including a phylogenetic correlation matrix as a random effect using the statistical package ape [27]. The phylogenetic correlation matrix was derived from a phylogeny constructed from information by Jetz et al. [22]. As we include species from multiple taxonomic families in our analysis, the Jetz et al. [22] phylogeny is probably a satisfactorily accurate representation of the evolutionary relationships and genetic distance between the species. The models were written based on predictably important ecological variables and their interactions relevant to the elevational distribution of a species, such that each model represented a specific hypothesis. Each model had the same structure ([Hb] ∼ Predictor 1 + Predictor 2 + Predictor 1 × Predictor 2 + (1|Species)). The variables used in each model are given in table 1. For predictor variables that were highly correlated (r > 0.6) [28], we retained those variables that were ecologically relevant to hypoxia physiology; for example, we retained ‘upper limit’ rather than ‘lower limit’ of the elevational distribution of a species. A complete description of models is given in table 1. For all models, we included ‘species’ as a random intercept effect. We fitted the linear mixed models using the lmekin function of the coxme package [29], and model selection was done using the MuMIn package [30]. All analyses were done in the software package R v. 3.2.1 [31].
Table 1.
Results of model selection of linear mixed models to investigate specific hypotheses predicting Hb concentration. Hct, haematocrit; EM, elevational migrant (1, 0); mass, mass of the individual (in grams); range, elevational range (in metres); range position, proportion of range where sampled; elevation, elevation at which sampled; upper limit, highest limit of elevational distribution. X indicates predictors used as fixed effects in the model. Species was used as a random intercept effect in all models. All models include an interaction between the two variables in the model. Models are arranged from models showing highest fit to models showing lowest fit.
| model no. | Hct | EM | mass | range | elevation | upper limit | range position | d.f. | ΔAICc |
|---|---|---|---|---|---|---|---|---|---|
| 3 | X | X | 6 | 0 | |||||
| 4 | X | X | 6 | 12.33 | |||||
| 6 | X | X | 6 | 14.39 | |||||
| 2 | X | X | 6 | 17.73 | |||||
| 9 | X | X | 6 | 22.2 | |||||
| 1 | X | X | X | X | X | X | X | 11 | 27.66 |
| 5 | X | X | 6 | 33.84 | |||||
| 10 | X | X | 6 | 35.81 | |||||
| 7 | X | X | 6 | 37.22 | |||||
| 8 | X | X | 6 | 37.79 |
3. Results
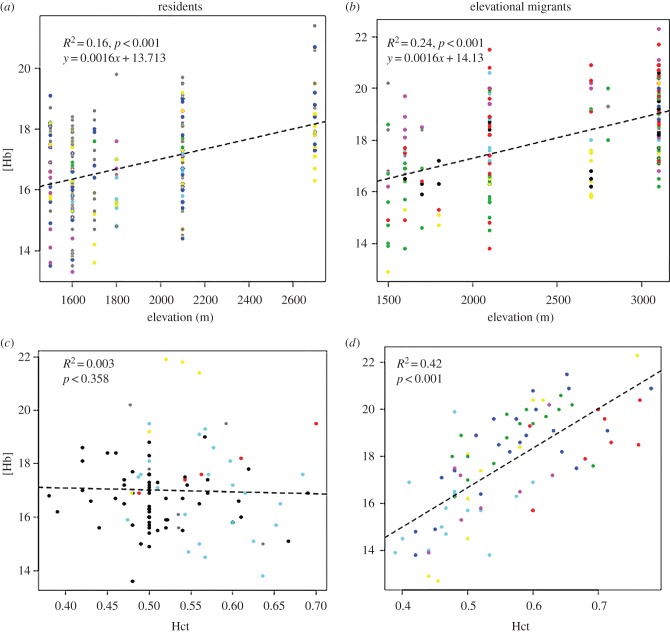
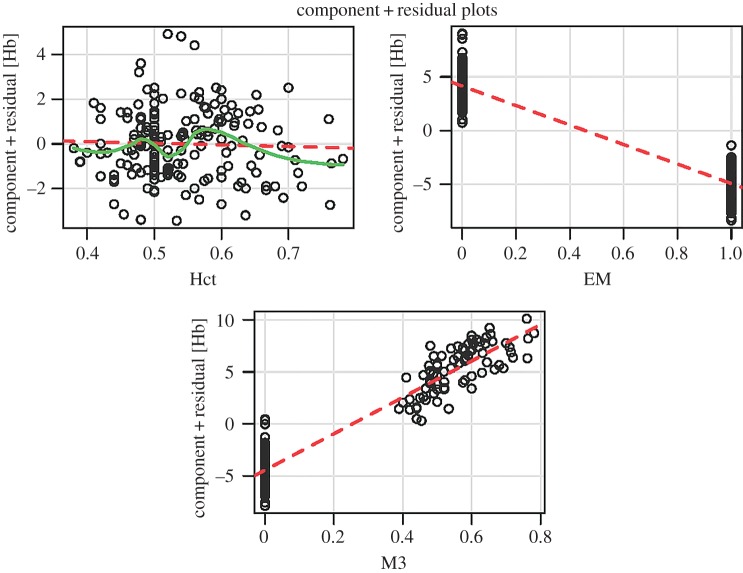
There was a significant positive relationship between [Hb] and elevation in both elevational migrants (R2 = 0.24, p < 0.001) and high-elevation residents (R2 = 0.16, p < 0.001; figure 2a,b). However, we found that the strength of the association between [Hb] and Hct is a function of the migratory status of a species. Although elevational migrants exhibit a strong positive relationship between [Hb] and Hct (R2 = 0.42, p < 0.001), there was no significant relationship between the two variables in resident species (R2 = 0.003, p > 0.35), suggesting an uncoupling of [Hb] and Hct in resident birds (figure 2c,d). Moreover, MCHC was significantly higher in sedentary residents than elevational migrants (t = 3.2076, d.f. = 45, p < 0.01), suggesting that erythrocytes of high-elevation residents contain more haemoglobin than those of elevational migrants. In the breeding season linear mixed model analysis, controlling for phylogeny, the best-fitting model (model 3, Hb ∼ Hct + EM_Hct × EM + (1|Species)) describes variation in [Hb] as a product of Hct, EM status (1, 0) and an interaction between Hct and EM (table 1). This model was weighted appreciably higher than any other model (table 1). Hct and EM have weak effects, but the interaction between Hct and EM has statistically significant positive effect on the variation of [Hb] (figure 3). This result, coupled with the differences in the strength of the relationship between [Hb] and Hct in migrants and residents, suggests that residents exhibit a common and functionally different mechanism for regulating [Hb] than migrant species (figure 2). In the non-breeding season, the global model (including all fixed effects) is selected as the top model in the linear mixed model analysis.
Figure 2.
Haemoglobin concentration [Hb] (g/dl) in (a) residents and (b) elevational migrants increases with elevation. (c) [Hb] is not correlated with haematocrit (Hct) in residents, but (d) is highly correlated in elevational migrants.
Figure 3.
Partial residual plots for top model in linear mixed model analysis [Hb ∼ Hct + EM + Hct × EM + (1|Species)]. M3 denotes the interaction factor between Hct and EM (elevational migration status). (Online version in colour.)
4. Discussion
Here, we show that although both high-elevation residents and elevational migrants modulate [Hb] to increase blood O2 carrying capacity in the face of environmental hypoxia (figure 2a,b), they achieve elevated [Hb] at high elevation through different means. The strong correlation between [Hb] and Hct in elevational migrants suggests retention of an ancestral strategy where lowland taxa increase [Hb] through upregulation of erythrocyte production (figure 2d) [32]. The lack of such a correlation in high-elevation resident species (figure 2c) suggests that species that consistently reside at high elevations regulate [Hb] independent of increases in Hct. Such a strategy would be particularly advantageous for highland residents as it would allow them to increase blood O2 carrying capacity without the concomitant increases in blood viscosity incurred by excessive erythropoiesis [33].
An increase in [Hb] can be brought about by several mechanisms other than increasing Hct [34]. Perhaps most important is to simply increase the [Hb] of individual erythrocytes (i.e. to increase the MCHC). Consistent with this expectation, we found that MCHC was significantly higher in resident species than elevational migrants residing at high elevation (t = 3.2076, d.f. = 45, p < 0.01). Together, our results suggest that high-elevation residents probably increase [Hb] through an increase in MCHC while elevational migrants increase [Hb] through an increase in Hct. An increase in the MCHC may lead to an increase in erythrocyte size, reducing oxygen diffusion to tissues [1,35], suggesting that there is likely to be a threshold to increase in MCHC. Highland mammals are therefore known to have smaller erythrocytes to increase oxygen transport than low-elevation conspecifics [15,36]. Owing to logistical reasons, we could not measure erythrocyte size in our study species.
In contrast to high-elevation residents, elevational migrants spend only a short period of time at high elevations every year. This life-history strategy may require the ability to fine-tune blood O2 carrying capacity throughout the year. A correlated increase in [Hb] and Hct is the most commonly documented acclimatization response to environmental hypoxia in birds and other vertebrates [7,19,37]. Hct exhibits a high degree of phenotypic flexibility in most vertebrates [15,38]. Thus, Hct-mediated changes in blood O2 carrying capacity may provide elevational migrants with the ability to fine-tune [Hb] throughout the year, and the increase in blood O2 carrying capacity brought about by increases in Hct might offset the costs of increased blood viscosity during the relatively short breeding seasons at high elevation.
Although migratory strategy was the principal predictor of variation in [Hb] in our linear mixed model analyses, the effect of migratory strategy was much more pronounced in the breeding season compared with the non-breeding season. The best-fitting model in the winter (non-breeding season) was the global model that included all the fixed-effect variables. This apparent discrepancy may be explained by the fact that all of the elevational migrants were sampled at low elevations (1000–2600 m) during periods of low physiological hypoxic stress, and therefore exhibiting low [Hb] and Hct values.
An important additional element of blood O2 carrying capacity is Hb–O2 affinity. Owing to difficult field logistics, we could not freeze blood samples to carry out this analysis. We therefore did not sequence the haemoglobin genes in these birds as functional data are essential to understand the phenotypic consequences of substitutions in haemoglobin genes [39]. Future studies should therefore carry out functional assays of Hb–O2 affinity and couple them with studies of sequence polymorphisms in haemoglobin genes [40,41] to better understand the mechanisms underlying the distinct strategies in [Hb] regulation between elevational migrants and high-elevation residents.
The extent to which hypobaric hypoxia might limit bird species distribution is not well understood [42]. A first step towards a general understanding of how bird species distribution might be restricted by hypoxia is to study common patterns in physiological responses in wild birds. Understanding interspecific variation in hypoxia tolerance is increasingly critical in light of climate change since hypoxia is unaffected by increasing temperatures and several montane species are predicted to show an upslope range expansion. Our study demonstrates that elevational migration, a common life-history strategy in montane birds, can explain interspecific differences in physiological strategies to cope with hypoxia. Although several Himalayan passerine species occur at elevations higher than our highest sampling site, the elevational range we sampled harbours much higher avian bird diversity than areas above 4000 m, both in the Himalayas and across the globe [43]. Furthermore, the upper elevations we did sample appear to be sufficient to induce hypoxic stress in birds as parallel genetic mutations to increase Hb–O2 affinity have evolved at elevations as low as 2000 m in Andean hummingbirds [40]. In the Himalayas, up to 65% of the species that breed at the highest elevations are elevational migrants [18,44] which thus make up a significant part of the bird community at these elevations in the breeding season. Our findings show that elevational migrants exhibit an erythropoietic response to environmental hypoxia that is typical of lowland species. This ancestral, erythropoietic response and its associated costs may affect the rate of upslope migration even if thermal conditions are favourable [32]. Thus, incorporating information on the specific physiological mechanisms that individual species employ to cope with hypoxia might be crucial for accurately estimating range shifts in high-elevation montane birds.
To the best of our knowledge, our study is the first to explore the erythropoietic response to hypoxia at the level of a bird community along an elevational gradient. This has revealed common strategies in the response to hypoxia strongly associated with the species' pattern of elevational movement. Several biotic and abiotic factors might simultaneously affect the elevational distribution of a species. For example, hypoxic stress might influence outcomes of biotic interactions such as interspecific competition, and rates of upslope range shifts in low-elevation birds might be affected by the interaction between such biotic and abiotic stressors. Interactive effects of covarying stressors as constraints on species distributions will require increasing attention if projections of species distributions are to be accurate [45]. Our findings demonstrate that strategies to cope with hypoxia are associated with life-history traits and might be generalizable across species to yield predictions for other high mountain systems like the Andes, where movement patterns of birds are more poorly understood and more difficult to study.
Supplementary Material
Acknowledgements
We thank Prof. Shou-Hsien Li and two anonymous reviewers for valuable inputs that significantly enhanced the quality of the manuscript. We would like to thank Wesley Hochachka for help with statistical analyses, and Christopher Witt, Farah Ishtiaq, Jennie Miller and Dhananjai Mohan, for scientific inputs. We thank Harish Maithani, Vijay Ramesh, Pratik Joshi, Manon Munoz, Hannah Bensch, Soham Dixit, Viral Joshi, Sartaj Ghuman, Balwant Negi, Chris Bowden, Rekha Warrier, Supriya K., Himani Nautiyal, Shailee Shah, Devathi Parshuram, Megha Rao, Lauren Henelly, Pramod Bisht, the Wildlife Institute of India and the Uttarakhand Forest Department for fieldwork and logistical help. We thank the Bird Population Studies and Conservation Science lab groups at the Cornell Lab of Ornithology for constructive criticism. Bird illustrations were reproduced with permission from the Handbook of the Birds of the World Alive online, Lynx Edicions, Barcelona.
Ethics
Appropriate permissions were obtained from the Uttarakhand State (India) Forest Department for sampling of birds. Conventional mist-netting protocols were used during sampling.
Data accessibility
All the datasets used in this paper are available as the electronic supplementary material.
Authors' contributions
S.B. conceived and designed the study, acquired data, carried out the analysis and drafted the manuscript. A.A.D. and Z.A.C. designed the study and developed the manuscript. V.B.M. designed the study, acquired data and developed the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was financially supported through funds from the Athena Fund of the Cornell Lab of Ornithology, Atkinson Centre Sustainable Biodiversity Fund, the Sigma Xi Grants-in-Aid for Research, the Cornell Lab of Ornithology Research Fellowship, the Halberstadt Graduate Fellowship and the Mary Hartshorne Endowment.
References
- 1.Monge C, Leon-Velarde F. 1991. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol. Rev. 71, 1135–1172. [DOI] [PubMed] [Google Scholar]
- 2.Badyaev AV, Ghalambor CK. 2001. Evolution of life histories along elevational gradients: trade-off between parental care and fecundity. Ecology 82, 2948–2960. ( 10.1890/0012-9658(2001)082%5B2948:EOLHAE%5D2.0.CO;2) [DOI] [Google Scholar]
- 3.Price T. 1991. Morphology and ecology of breeding warblers along an altitudinal gradient in Kashmir, India. J. Anim. Ecol 60, 643–664. ( 10.2307/5303) [DOI] [Google Scholar]
- 4.Storz JF. 2010. Genes for high altitudes. Science 329, 40–41. ( 10.1126/science.1192481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake CI, Banchero N. 1985. Effects of cold and hypoxia on ventilation and oxygen consumption in awake guinea pigs. Respir. Physiol. 61, 357–368. ( 10.1016/0034-5687(85)90078-7) [DOI] [PubMed] [Google Scholar]
- 6.Costa LE, Mendez G, Boveris A. 1997. Oxygen dependence of mitochondrial function measured by high-resolution respirometry in long-term hypoxic rats. Am. J. Physiol.-Cell Physiol. 273, C852–C858. [DOI] [PubMed] [Google Scholar]
- 7.Moore LG, Niermeyer S, Zamudio S. 1998. Human adaptation to high altitude: regional and life-cycle perspectives. Am. J. Phys. Anthropol. 107, 25–64. ( 10.1002/(SICI)1096-8644(1998)107:27%2B%3C25::AID-AJPA3%3E3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 8.Beall CM, et al. 2010. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl Acad. Sci. USA 107, 11 459–11 464. ( 10.1073/pnas.1002443107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheviron ZA, Brumfield RT. 2012. Genomic insights into adaptation to high-altitude environments. Heredity 108, 354–361. ( 10.1038/hdy.2011.85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott GR. 2011. Elevated performance: the unique physiology of birds that fly at high altitudes. J. Exp. Biol. 214, 2455–2462. ( 10.1242/jeb.052548) [DOI] [PubMed] [Google Scholar]
- 11.Sakai A, et al. 2003. Cardiopulmonary hemodynamics of blue-sheep, Pseudois nayaur, as high-altitude adapted mammals. Jpn. J. Physiol. 53, 377–384.( 10.2170/jjphysiol.53.377) [DOI] [PubMed] [Google Scholar]
- 12.Crowell JW, Smith EE. 1967. Determinant of the optimal hematocrit. J. Appl. Physiol. 22, 501–504. [DOI] [PubMed] [Google Scholar]
- 13.Schuler B, Arras M, Keller S, Rettich A, Lundby C, Vogel J, Gassmann M. 2010. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc. Natl Acad. Sci. USA 107, 419–423.( 10.1073/pnas.0912924107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velguth KE, Payton ME, Hoover JP. 2010. Relationship of hemoglobin concentration to packed cell volume in avian blood samples. J. Avian Med. Surg. 24, 115–121. ( 10.1647/2008-042.1) [DOI] [PubMed] [Google Scholar]
- 15.Tufts DM, Revsbech IG, Cheviron ZA, Weber RE, Fago A, Storz JF. 2013. Phenotypic plasticity in blood-oxygen transport in highland and lowland deer mice. J. Exp. Biol. 216, 1167–1173. ( 10.1242/jeb.079848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowell JW, Ford RG, Lewis VM. 1959. Oxygen transport in hemorrhagic shock as a function of the hematocrit ratio. Am. J. Physiol. Content 196, 1033–1038. [DOI] [PubMed] [Google Scholar]
- 17.Villafuerte FC, Cardenas R, Monge CC. 2004. Optimal hemoglobin concentration and high altitude: a theoretical approach for Andean men at rest. J. Appl. Physiol. 96, 1581–1588. ( 10.1152/japplphysiol.00328.2003) [DOI] [PubMed] [Google Scholar]
- 18.Dixit S, Joshi V, Barve S. 2016. Bird diversity of the Amrutganga Valley, Kedarnath, Uttarakhand, India with an emphasis on the elevational distribution of species. Check List 12, 1874 ( 10.15560/12.2.1874) [DOI] [Google Scholar]
- 19.Carey C, Morton ML. 1976. Aspects of circulatory physiology of Montane and lowland birds. Comp. Biochem. Physiol. A Physiol. 54, 61–74.( 10.1016/S0300-9629(76)80073-4) [DOI] [PubMed] [Google Scholar]
- 20.Alice Boyle W, Sandercock BK, Martin K. 2015. Patterns and drivers of intraspecific variation in avian life history along elevational gradients: a meta-analysis. Biol. Rev. 91, 469–482. ( 10.1111/brv.12180) [DOI] [PubMed] [Google Scholar]
- 21.Boyle WA. 2008. Can variation in risk of nest predation explain altitudinal migration in tropical birds? Oecologia 155, 397–403. ( 10.1007/s00442-007-0897-6) [DOI] [PubMed] [Google Scholar]
- 22.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 23.del Hoyo J, Elliot A, Sartagal J, Christie DA, De Juana E. 2014. Handbook of the birds of the world alive. Barcelona, Spain: Lynx Edicions; See www.hbw.com (accessed 30 October 2014). [Google Scholar]
- 24.Chappell MA, Bachman GC, Odell JP. 1995. Repeatability of maximal aerobic performance in Belding's ground squirrels, Spermophilus beldingi. Funct. Ecol. 9, 498–504.( 10.2307/2390015) [DOI] [Google Scholar]
- 25.Wolak ME, Fairbairn DJ, Paulsen YR. 2012. Guidelines for estimating repeatability. Methods Ecol. Evol. 3, 129–137. ( 10.1111/j.2041-210X.2011.00125.x) [DOI] [Google Scholar]
- 26.Ali S, Ripley SD. 1983. Handbook of the birds of India and Pakistan, compact edition Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 28.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. See https://cran.r-project.org/package=lme4.
- 29.Therneau T. 2012. coxme: mixed effects Cox models. R package version 2.2-3. See https://cran.r-project.org/web/packages/coxme/coxme.pdf. [Google Scholar]
- 30.Barton K. 2011. MuMIn: Multi-model inference. R package version 1.0. 0. See http://cran.r-project.org/package=MuMin. [Google Scholar]
- 31.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 32.Storz JF, Scott GR, Cheviron ZA. 2010. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125–4136. ( 10.1242/jeb.048181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beall CM. 2002. Tibetan and Andean contrasts in adaptation to high-altitutde hypoxia. In Oxygen sensing (eds S Lahiri, NR Prabhaker, RE Forster II), pp. 63–74. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez J-M, Folkow LP, Blix AS. 2007. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu. Rev. Physiol. 69, 113–143. ( 10.1146/annurev.physiol.69.031905.163111) [DOI] [PubMed] [Google Scholar]
- 35.Canals M, Donoso C, Figueroa D, Sabat P. 2007. Pulmonary hematological parameters, energetic flight demands and their correlation with oxygen diffusion capacity in the lungs. Rev. Chil. Hist. Nat. 80, 275–284. ( 10.4067/S0716-078X2007000300002) [DOI] [Google Scholar]
- 36.Hawkey CM, Bennett PM, Gascoyne SC, Hart MG, Kirkwood JK. 1991. Erythrocyte size, number and haemoglobin content in vertebrates. Br. J. Haematol. 77, 392–397. ( 10.1111/j.1365-2141.1991.tb08590.x) [DOI] [PubMed] [Google Scholar]
- 37.Borras A, Cabrera J, Senar JC. 2010. Hematocrit variation in response to altitude changes in wild birds: a repeated-measures design. Condor 112, 622–626. ( 10.1525/cond.2010.090113) [DOI] [Google Scholar]
- 38.Birchard GF. 1997. Optimal hematocrit: theory, regulation and implications. Am. Zool. 37, 65–72. ( 10.1093/icb/37.1.65) [DOI] [Google Scholar]
- 39.Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF. 2013. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340, 1324–1327. ( 10.1126/science.1236862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Projecto-Garcia J, et al. 2013. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc. Natl Acad. Sci. USA 110, 20 669–20 674. ( 10.1073/pnas.1315456110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. 2010. Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213, 2565–2574. ( 10.1242/jeb.042598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jankowski JE, Londoño GA, Robinson SK, Chappell MA. 2013. Exploring the role of physiology and biotic interactions in determining elevational ranges of tropical animals. Ecography 36, 001–012. ( 10.1111/j.1600-0587.2012.07785.x) [DOI] [Google Scholar]
- 43.Elsen PR, Tingley MW. 2015. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Change 5, 772–776. ( 10.1038/nclimate2656) [DOI] [Google Scholar]
- 44.Somveille M, Manica A, Butchart SH, Rodrigues AS. 2013. Mapping global diversity patterns for migratory birds. PLoS ONE 8, e70907 ( 10.1371/journal.pone.0070907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham CH, et al. 2014. The origin and maintenance of montane diversity: integrating evolutionary and ecological processes. Ecography 37, 711–719. ( 10.1111/ecog.00578) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the datasets used in this paper are available as the electronic supplementary material.