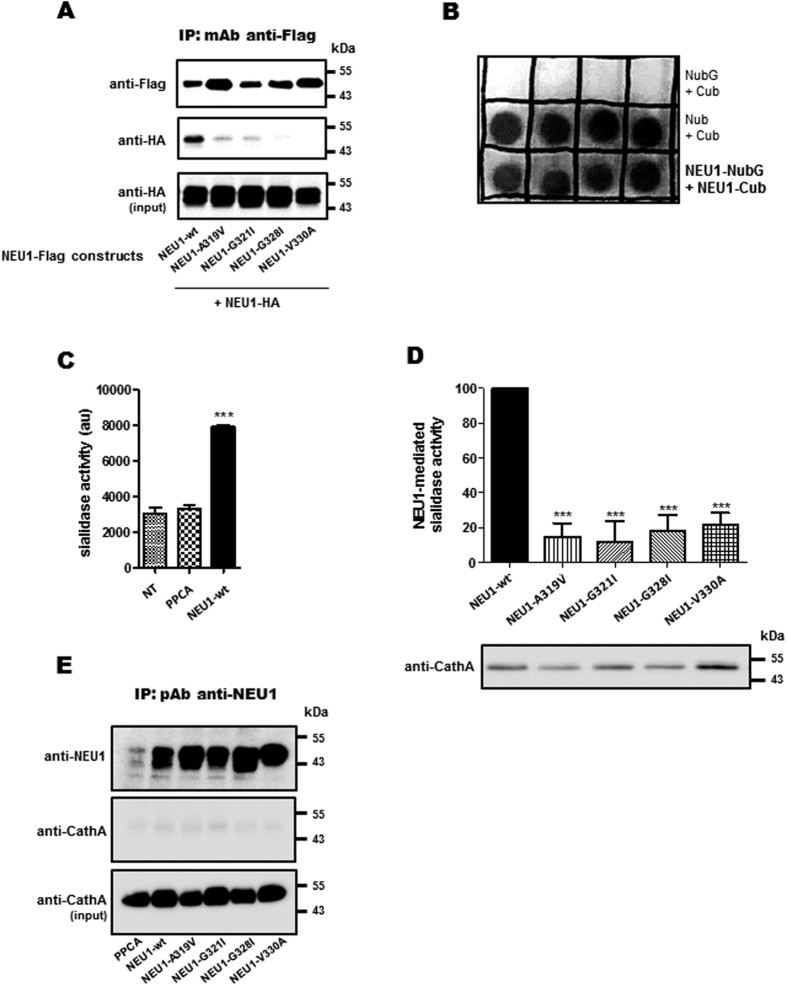
Figure 8. Point mutations in NEU1/TM2 strongly affect NEU1 dimerization and membrane sialidase activity.
(A) The NEU1-Flag constructs were immunoprecipitated from crude membrane fractions of co-transfected COS-7 cells using a mouse monoclonal anti-Flag antibody and co-immunoprecipitated NEU1-HA was monitored by western blot using a rabbit monoclonal anti-HA antibody. The figure is representative of 3 independent experiments. (B) Direct interaction between NEU1 monomer was measured by split-ubiquitin yeast two-hybrid screen. Yeast cells were transformed with NubG and Cub (negative control), Nub and Cub (positive control) or NEU1-NubG and NEU1-Cub constructs. Yeast growth was challenged on minimal growth medium depleted of Trp and Leu by spotting four independent transformants on the different media. (C,D) Sialidase activity was measured in crude membrane fractions of COS-7 cells co-transfected with the different NEU1 constructs and PPCA (1:2). 50 μg/well of proteins were incubated with 200 μM of 2′-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic acid substrate for 2 h at 37 °C in MES 20 mM, pH 4.5. (D) Sialidase activity was expressed as normalized NEU1-mediated sialidase activity; e.g. sialidase activity mediated by transfected NEU1 (wild type or mutants) over their expression levels revealed by polyclonal anti-NEU1 antibody. Results are expressed as mean ± SEM of 3 independent experiments, each run in duplicate and normalized to NEU1 wt. ***p < 0.001. (E) The NEU1 constructs were immunoprecipitated from crude membrane fractions of co-transfected COS-7 cells using a rabbit polyclonal anti-NEU1 antibody and co-immunoprecipitated PPCA was monitored by western blot using a mouse monoclonal anti- cathepsin A.