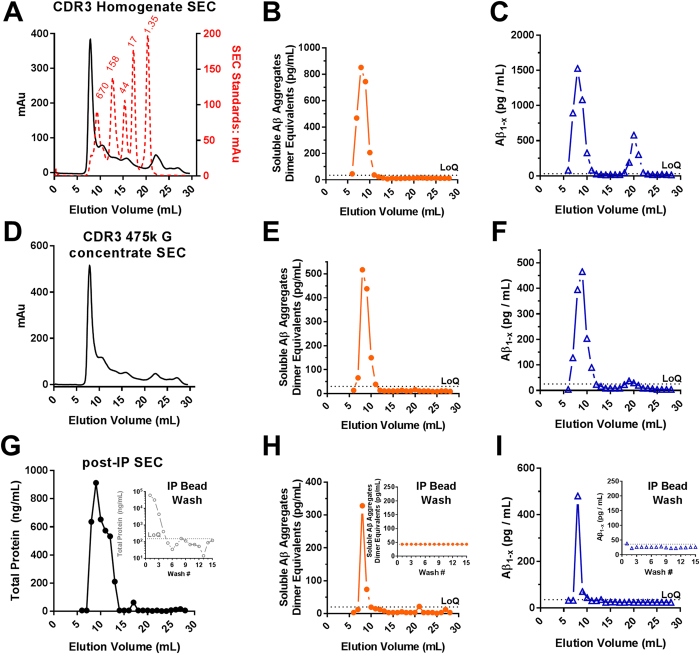
Figure 3. Enrichment and preservation of soluble high molecular weight Aβ aggregates from human AD brain following differential centrifugation and immunoprecipitation.
(A) Size exclusion chromatography (SEC) total protein profile (measured by absorption) of a CDR3 tissue homogenate prepared in 1xPBS containing 0.45% CHAPS; overlay with Bio-Rad gel filtration standards (red) with molecular weight in kDa indicated (red text). (B,C). Soluble Aβ aggregate and Aβ1−x assays on SEC fractions of original lysates. LoQ: limit of quantitation. (D) SEC total protein profile of the sucrose cushion fraction after ultracentrifugation at 475,000× g. (E,F) Soluble Aβ aggregate and Aβ1−x assays on SEC fractions of sucrose cushion fraction after ultracentrifugation at 475,000× g, demonstrating the preservation of soluble Aβ aggregates and separation from monomers. (G) SEC total protein profile (measured by NanoOrange) of the 475,000× g sucrose cushion fraction followed by immunoprecipitation (IP), washing, and elution with ammonium hydroxide. (H,I) Soluble Aβ aggregate and Aβ1−x assays on eluted high molecular weight soluble Aβ aggregates. Insets: Quantification of protein (note log scale in panel G), soluble Aβ aggregates, and Aβ1−x in the immunoprecipitation bead washes.