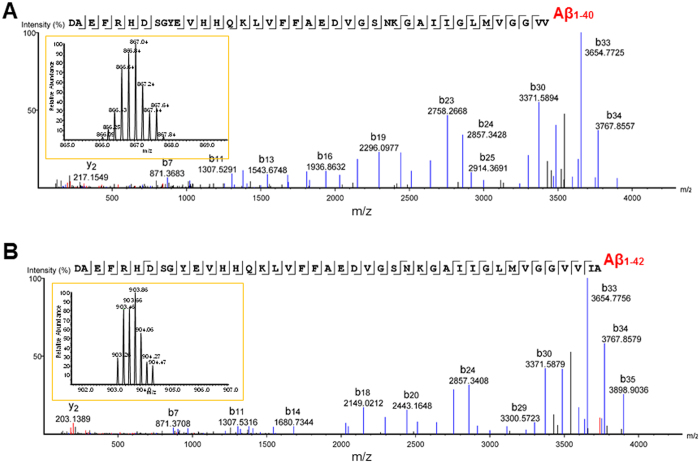
Figure 8. Mass spectrometry of soluble Aβ aggregates from human AD brain.
(A) Liquid chromatography-tandem mass spectrometry (LC-MS/MS) spectrum for undigested, full-length Aβ1-40. Each peak represents an ion fragmented from Aβ1-40, with peaks labeled ‘b’ representing N-terminal fragment ions and peaks labeled ‘y’ representing C-terminal fragment ions. The numbers indicate measured mass/charge ratio (m/z). The single letter amino acid code across the top indicates the de novo sequence identified by mass spectrometry, which matches the amyloid precursor protein sequence corresponding to Aβ1-40. The line breaks between amino acids indicate a cleavage of the amide bond between two adjacent amino acids producing fragment ions. The lines below each amino acid indicate a detected ‘b’ ion, and lines above indicate a detected ‘y’ ion. Inset: isotopic envelope for the +5 charged, full-length Aβ1-40: the peaks are spaced 0.2 daltons apart at z = +5 because the naturally occurring isotopes (e.g. 13C and 15N) differ by 1 dalton. For the +5 ion, the observed m/z was 866.4351 (theoretical m/z = 866.4370), which was −2.1 parts per million (ppm) error from the theoretical mass of Aβ1-40. (B) Spectrum for full length Aβ1-42. For the +5 ion, the observed m/z was 903.2623 (theoretical m/z = 903.2612), which was 1.2 ppm error from the theoretical mass of Aβ1-42.