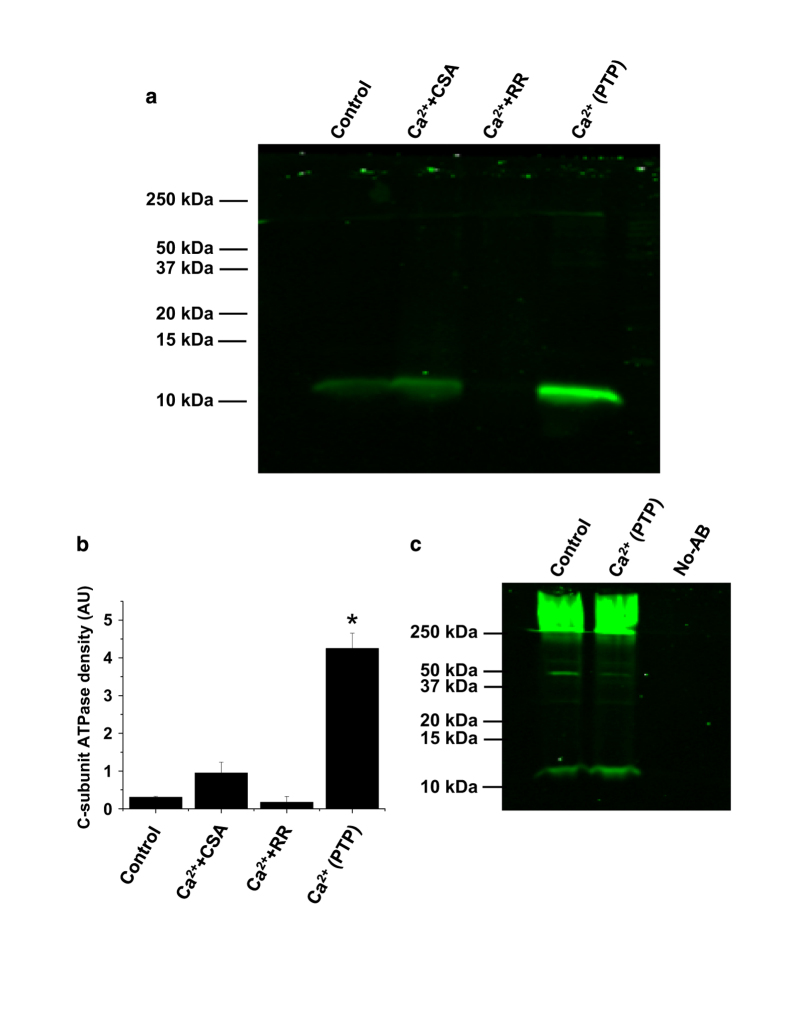
Figure 2.
mPTP activation increases C-subunit content of the channel-forming fraction (chloroform extractable C-subunit). (a) Western blot using C-subunit-specific antibodies shows that mPTP activation in isolated mitochondria (+Ca2+) increases the amount of C-subunit associated with channel-forming 'PTP pore' fraction. Note that the amount of complex is dramatically decreased if mPTP is inhibited with 5 μM Ruthenium Red (Rut Red) or 1 μM CSA despite the presence of the same amounts of Ca2+ (200 μM). The samples for analysis were purified using chloroform extraction protocol as described in Pavlov et al.12 and identical to the samples used for mPTP monitoring in Figure 1. (b) Densitometry analysis of western blots (as in a) from three independent experiments (*P<0.01 t-test). (c) Western blot of C-subunit immunoprecipitated from the total mitochondrial lysate using C-subunit antibodies shows similar total amounts of C-subunit in different samples. Note high molecular bands presumably formed by oligomers of the C-subunit.