Abstract
We have developed a method that for the first time allowed us to synthesize silica particles in 20 minutes in a sol-gel preparation. Therefore, it is critically important to understand the synthesis mechanism and kinetic behavior to achieve higher degree of fine tuning ability in synthesis. In this study, we have employed our ability to modulate the physical nature of the reaction medium from sol-gel to emulsion have allowed us to halt the reaction at a particular time; this has allowed us to precisely understand the mechanism and chemistry of the silica polymerization. The synthesis medium is kept quite simple with tetraethyl orthosilicate (TEOS) as precursor in equi-volumetric ethanol-water system and sodium hydroxide as a catalyst. Synthesis is performed under ambient conditions at 20 °C for 20 minutes followed by phasing out the unreacted TEOS and polysilicic acid chains by their emulsification with supersaturated water. We have also demonstrated that the developed particles in various sizes can be used as seeds for further particle growth and other applications. Luminol, a chemilumniscent molecule, has been entrapped successfully between the layers of silica and was demonstrated for the chemiluminescence of these particles.
Keywords: Silica particle, Synthesis, Nucleation, Polysilicic acid, Precipitation, NaOH, Stober, Phasing-out, TEOS
Graphical abstract
Introduction
Silica nanoparticles (SiNPs) have been widely used in electronics, photonics, biosensors, and drug discovery.1-4 Albeit their far reaching applications, an understanding of the basic mechanism of nanoparticle formation is a critical missing link. Phenomenon like hollowing of the particles in aqueous system presents explanatory challenges.5 Typically, nucleation, particle growth, and ripening are controlled through a series of well manipulated chemical reactions for synthesizing fine-tuned particle sizes.6-17 However, almost all the kinetically-controlled particle synthesis methods are time-limited and require longer durations, typically 7-24 h.18-22 We, on the other hand, have employed a very convenient approach to study particle formation behaviour on a timescale of few minutes. Unlike recent reports in catalyst-free or mild catalyst synthesis18,21-23, which require several hours to synthesize particles via a very slow process, we have employed sodium hydroxide catalyst as reported by Bhakta et al. (2016) and observed significant enhancementin the silica polymerization and precipitation process thus eliminating the rate-limitedness of our synthesis process and particle seeding behavior.24 Taking advantage of poor miscibility of TEOS in water25 to phase out unreacted silica from solution with a super saturated aqueous medium, we have precisely controlled the nanoparticle formation and reaction kinetics without altering additional dependent variables, such as temperature, TEOS concentration, and catalyst amount (Scheme 1). Thus, we can summarize it as the first report based on the TEOS depletion in the medium for controlled synthesis and growth of silica nuclie for which we have tried to understand the mechanism of nucleation and growth employing HR-LVSEM complemented with dynamic light scattering (DLS). This is in contrast to other reports employing advanced analytical approaches for studying nanoparticle synthesis via slow kinetics, which include but are not restricted to Si-NMR26, dissolution kinetics with small angle X-ray scattering (SAXS)27,28, and cryo-TEM18,29. Our approach is also bio-friendly as ultra-low concentration of sodium is present in the system with minimal hazardous waste.
Scheme 1.
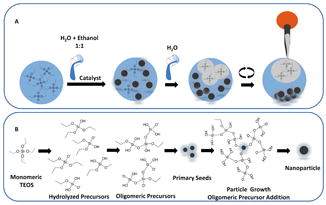
Mechanism of silica nucleation, seed development, and particle growth. (A) represents a new approach to control the particle development as a function of phasing out TEOS precursor from ethanol solution by supersaturating the reaction medium with water. Addition of excess water will immediately moves TEOS from solution phase to emulsion thus stopping the particle growth immediately. The phased out TEOS then can be removed by either simple pipetting, centrifugation, or both. (B) depicts the hydrolysis and condensation of TEOS taking place in solution phase. TEOS hydrolyzed to all its probabilistic derivatives which later condense to form oligomeric species followed by formation of primary seeds. The condensate oligomers will further precipitate over the seed contributing to the particle growth.
Results and Discussion
In the proposed approach silica precursor (TEOS) was allowed to burst nucleate under mild basic condition with sodium (Na) as counter ion. This condition favours the condensation of TEOS over the hydrolysis to form three dimensional cyclic polysilicic acid chain networks.6 These 3-dimensional networks in an equi-volumetric ethanol-water medium get aggregated after reaching supersaturation (critical aggregation concentration; CAC) thus generating nanoparticles. The role of ethanol is important in the medium as it enhances the TEOS solubility during the polymerization in the medium and significantly lowers the otherwise high hydration potential of water allowing CAC to be reached in much less time.21 This has accelerated the nanoparticle formation in our case as evident from our findings where we were able to synthesize particles within minutes at specific ethanol-water ratio in the reaction medium (Figure 1; Supplementary Note 1). Considering the consumption behavior of TEOS and hydroxyl (OH−) in solution (Supplementary Table 1 & 2) our findings were in agreement with the theory of burst nucleation mechanism16,30. As evident, the hydroxyl ionconcentration substantially decreased within first 20 min. This may indicate fast hydrolysis of TEOS that will generate monomeric and oligomeric silicic acid species thus, decreasing the pH of the solution. At 30 min the hydroxyls in the system increased sharply as an indicative of a significant decrease in the amount of silicic acid attributed to polymerization. Afterwards, OH− concentration steadied over the time range between 30-75 min that may be suggestive of silica densification over that period.
Fig 1.
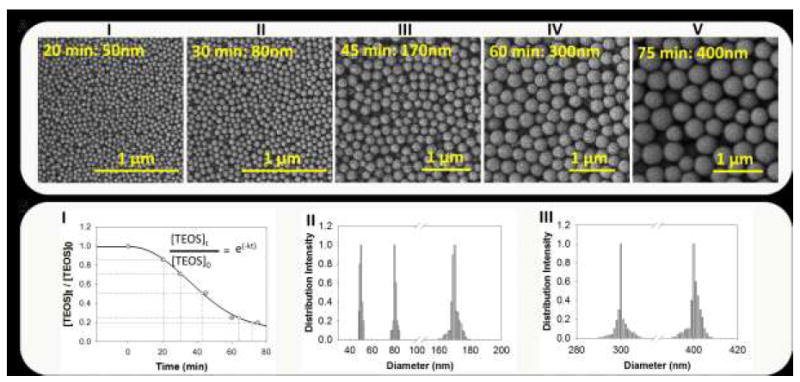
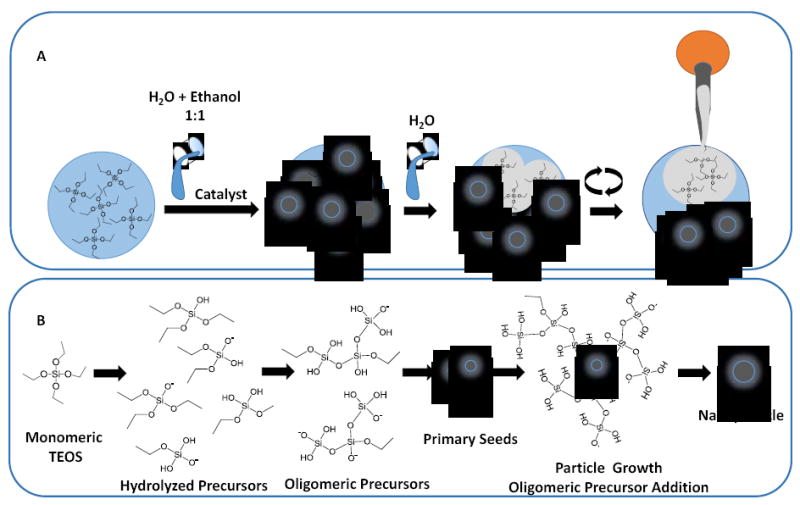
Illustration of the particle condensation and size growth. (A) Controlling the particle growth according to the scheme 1A. Following fast reaction kinetics in conjunction with TEOS phasing out, particles in a range of 50-400nm were formed with high monodispersity. Each particle is listed with the duration of synthesis, median diameter in nm, and scale of imaging. (B) I. The particle formation kinetics as a function of TEOS recovered in the emulsion phase; II & III. depict the particle size distribution across the median value as they appear in sequence in panel A.
In our approach, rather than changing variables, we simply stopped the densification process by removing unreacted TEOS, as depicted in Scheme 1, via increasing the water content in the system. Due to poor miscibility of TEOS, water will phase it out of the ethanolic medium.25 Therefore, by regulating the water content in the medium we were able to control the extent of nucleation and the recovery of TEOS (Supplementary Note 2, Supplementary Table 4 & 5). By employing this strategy we have obtained specific nanoparticle sizes (50-400 nm) without regulating the other reaction variables (Figure 1A) with considerably high monodispersity (Figure 1B). Here, our main focus is to understand mechanism of nanoparticle growth and its impact on size homogeneity. Typically, it is proposed that nanoparticle formation follows the classical nucleation and growth model, which emphasizes on a single burst nucleation in order to obtain uniform size distribution.16 Considering this theory, the monodispersity is a function of rapid polymerization followed by hasten precipitation that dramatically increases the number of nuclei and limit the densification at surface due to depleted precursor concentration.13,18,21 Catalytic and thermal regulation of the process to increase the number of nuclei in the medium for reducing the nanoparticle size has been proposed on several occasions.9,19,22,31 We based our synthesis method on the previous findings to obtain fast nucleation and employed Na as catalyst, which also serves as counter ion in our system. This has enhanced the reaction kinetics respective to ethanol and ethanol-water mediums, as evident from particle sizes (Figure 2). We found that the TEOS consumption was fastest within 20 min of the reaction and decreased sharply over the remaining reaction duration (Supplementary Table 3). This was consistent with the particle size patterns from the electron microscopy imaging. Such kinetic behavior is attributed to the continuously depleting TEOS concentration in the reaction (Figure 1 BI) and is consistent with the predicted number of nanoparticles in each system as summarized in Supplementary Table 6. As the TEOS consumption rate decreased over 20-75 min duration, the particle number decreased with increasing reaction time (Supplementary Note 3; Supplementary Table 6). We are of the opinion that, at the beginning, nucleation should be equal in all the sets for 20-75 min duration but decreasing TEOS concentration in the system might have restricted any further nucleation and precipitation. However, as the reaction was allowed to continue longer, the oligomeric TEOS nuclei, which failed to grow further under depleted TEOS environment, will start precipitating on the surface of the already formed nuclei to give rise to bigger particles, which rationally explains the decreasing particle number with the increasing reaction time. We deduced from these findings that until the nuclei reach a critical size and mass, silica nuclei is dynamic between both formation and deformation processes. We opine that this interplay of dynamics is critical for grafting oligomeric silica nuclei to precipitate and form bigger particles, which is collinear with the classical approach as reported previously21 but also indicates toward an alternative possible mechanism of growth where densifications would be via fusion of several silica nuclei rather than the addition of monomeric or oligomeric units, which has been recently reported18.
Fig 2.
Densification behaviour of TEOS on seed particles imaged in ‘A’. The particle growth was observed under three different catalyst conditions viz. ethanolic solution (B), equi-volumetric ethanol-water solution (C), and medium conditions as in ‘C’ with NaOH as catalyst. Seeding was performed over 3 and 12 h durations for ‘B’ and ‘C’ while only 3 h for NaOH. Each panel is illustrated with particle size and duration of seeding. As evident, each method employs different kinetics of TEOS surface addition as a function of medium over same reaction durations.
After allowing this reaction to happen for certain period of time, the TEOS was then phased-out from the equi-volumetric ethanol:water medium by increasing the water content of the medium. TEOS in water forms an emulsion due to poor miscibility. We have used this to our favour for removing TEOS from the reaction medium. In different batches, after allowing the reaction to take place for designated times, water was added to the reaction medium to achieve 15-fold volumetric excess against ethanol. This will phase out different TEOS amount overtime and thus will remove any traces of unreacted TEOS along with small polysilicic chains that failed to precipitate by simply collecting out the immiscible TEOS fraction from the medium (Supplementary Note 2; Supplementary Table 5). This practically exhausted the precursor in solution halting the polymerization and precipitation resulting in the development of a range of different particle sizes for specific reaction duration. In addition, water will saturate the surface of already formed particles thus stabilizing them, as evident from the zeta potential (-45 to -60 mV). An added advantage of this synthesis approach is that appropriate manipulation of water and ethanol contents in the medium allowed us to continue the reaction with the convenience of stopping the reaction as desired for sampling.
We now have extensive control on the nanoparticle size allowing us to probe the reaction behaviour of particle growth. To further explore the mechanism, we added TEOS in an ethanolic solution saturated with 50nm particles as seed (Figure 2A). Saturation of the solution with seed particles has allowed us to reduce the burst nucleation following the initial hydrolysis/condensation of additional TEOS. We speculate it to be mainly governed by an interplay between hydroxyls on particle surface and that of the ethanol. This limits the nucleation kinetics favouring addition of the hydrolyzed silicates on the seed surface. Therefore, we expected the addition of TEOS on the particle surface as oligomeric species, which is in agreement to the several previously proposed models31,32. We found a time-limited addition of polymers on nanoparticle surface and have observed formation of 51±1nm particles over 3 h duration (Figure 2B). This is equivalent to a TEOS monolayer (TEOS molecular diameter is ~0.95nm) and is in close approximation of the previous silanization models15,16,18,21. Further, we have obtained particles of 80±4nm if the reaction was allowed to continue for 12 h (Figure 2B). On the contrary, the particle growth was fast for an equi-volumetric ethanol:water medium and we obtained 70±5nm particles for the first 3 h reaction reaching to 150±13nm in 12 h (Figure 2C). However, later reaction resulted in higher polydispersity although the particles were very stable with zeta measuring between – 40 to −50mV. In this case, relatively higher polydispersity compared to the ethanol medium may be attributed to an increase in the hydrolysis of TEOS in presence of water. We speculate that this may develop new nucleation sites in addition to the seed nanoparticles. Once the system is TEOS depleted the new silica nuclei may precipitate on the growing seed particles in an uncontrolled manner thus increasing the polydispersity. If NaOH was added to a solution of TEOS mixed with 50nm seeds, the particles were highly polydispersed sizing between 150-450nm for the 3 h reaction (Supplementary Figure 1). An obvious interpretation could be disruption of ethanol-particle hydroxyl interactions due to the counter ion resulting in fast heterogeneous surface reaction. Conversely, polydispersity ceased significantly if NaOH was added prior to TEOS addition along with a below saturation nanoparticle solution; the average particle size for this reaction system was 510±8nm (Figure 2D). These findings indicate that polymerization on particle surface is a rate-limiting step and will require an appropriate catalyst to facilitate the seed growth via single mode with less polydispersity upon maturation.
Such surface reaction on the seed can be employed to entrap probe molecules for sensing applications. We have entrapped Luminol, a chemiluminescent probe, in the particles via formation of an ultra-thin protective silica layer on top (Figure 3A). The entrapped luminol have no noticeable decrease in its luminescence for its equivalent content in free solution (Figure 3B). A probabilistic mechanism is explained in Supplementary Figure 2. The application of this approach thus can be extended to entrap biomolecules and other particles to develop efficient probes for plasmonics, photonics, and energy.
Fig 3.
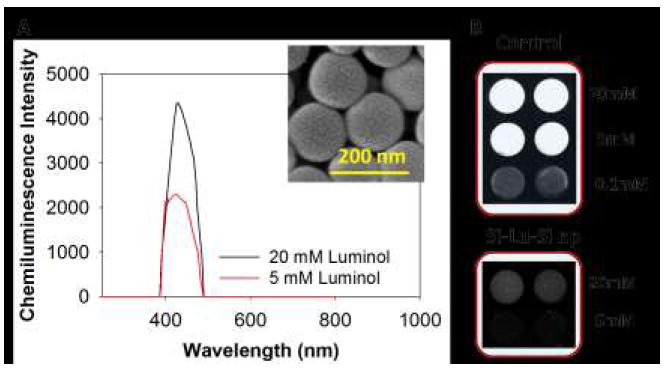
Entrapping luminol between the seed and the grafted silica on the seed. (A) Chemiluminescence intensities of luminol entrapped at two different concentrations under same conditions. Inset shows particles after entrapment of luminol. (B) The CCD image of luminol entrapped silica nanoparticles. Control is where free luminol was assayed with hydrogen peroxide in presence of horseradish peroxidase enzyme while Si-Lu-Si is luminol entrapped particles assayed under same conditions as the control.
Conclusions
Based on our understanding of the TEOS miscibility in water, we developed a rapid and facile approach to precisely control the particle growth and thus its size with the ability to manipulate the surface properties for further functionalization and particle growth. Experimental particle size and TEOS retrieval via water-mediate phasing out were in correlation with the theoretical analysis. We understood from this study that the polydispersity of nanoparticles in a synthesis process is mainly controlled by the synthesis kinetics following burst nucleation and reaction duration. A faster reaction will result in higher polydispersity if allowed to react longer and likewise. We have used these assemblies as seeds to entrap molecules of interest via sandwiching them between the seed and the silica assembled over the seed surface. The findings suggest that with appropriate understanding of silica particle growth mechanism we can manipulate size and surface properties for wide range of applications extending from biosensors to energy.
Supplementary Material
Acknowledgments
Authors acknowledge the financial support from the Green Emulsions, Micelles, and Surfactants Center (GEMS) at University of Connecticut and grants EB016707 and EB014586 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), NIH, USA for financial support of this work. Authors acknowledge electron microscopy facility at Clarkson University and FEI UCONN (University of Connecticut). SB would like to thank the FEI UCONN for a fellowship grant. SLS acknowledges support of the US Department of Energy, Basic Energy Sciences, Division of Chemical, Geological and Biological Science under grant DE-FGO2-86ER13622.A000.
Footnotes
Author Contribution
SB and CKD have conceived the idea. SB, CKD, and AK have contributed to the process development and optimization. CKD, JFR, and SLS have mentored the work and contributed intellectually. SB, CKD, AK, JFR, and SLS have performed data analysis along with writing the manuscript.
Conflict of Interest
Authors claim no conflict of interest for this work
Supporting Information
Experimental section; TEOS polymerization kinetics as Note 1; Phasing behaviour of TEOS in water as Note 2; Nanoparticle size prediction as Note 3.
References
- 1.Chen C, Wang H, Xue Y, Xue Z, Liu H, Xie X, Mai Y-W. Compos Sci Technol. 2016;128:207–214. [Google Scholar]
- 2.Roy S, Dixit CK, Woolley R, MacCraith BD, O’Kennedy R, McDonagh C. Langmuir. 2010;26:18125–18134. doi: 10.1021/la103212d. [DOI] [PubMed] [Google Scholar]
- 3.Dixit CK, Roy S, Byrne C, O’Kennedy R, McDonagh C. Analyst. 2013;138:6277–6281. doi: 10.1039/c3an01294h. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S. Nanomedicine Nanotechnol Biol Med. 2015;11:313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Zhang T, Ge J, Hu Y, Zhang Q, Aloni S, Yin Y. Angew Chem Int Ed. 2008;47:5806–5811. doi: 10.1002/anie.200800927. [DOI] [PubMed] [Google Scholar]
- 6.Brinker CJ. J Non-Crytalline Solids. 1988;100:31–50. [Google Scholar]
- 7.Bogush GH, Tracy MA, Zukoski CF. J Non-Cryst Solids. 1988;104:95–106. [Google Scholar]
- 8.Aubert T, Grasset F, Mornet S, Duguet E, Cador O, Cordier S, Molard Y, Demange V, Mortier M, Haneda H. J Colloid Interface Sci. 2010;341:201–208. doi: 10.1016/j.jcis.2009.09.064. [DOI] [PubMed] [Google Scholar]
- 9.Berendjchi A, Khajavi R, Yazdanshenas M-E. Iran J Org Chem. 2013;5:1079–1083. [Google Scholar]
- 10.Green D, Lin J, Lam Y-F, Hu MZ-C, Schaefer DW, Harris M. J Colloid Interface Sci. 2003;266:346–358. doi: 10.1016/s0021-9797(03)00610-6. [DOI] [PubMed] [Google Scholar]
- 11.Hartlen KD, Athanasopoulos APT, Kitaev V. Langmuir. 2008;24:1714–1720. doi: 10.1021/la7025285. [DOI] [PubMed] [Google Scholar]
- 12.Donatti DA, Ruiz AI, Vollet DR. Ultrason Sonochem. 2002;9:133–138. doi: 10.1016/s1350-4177(01)00120-1. [DOI] [PubMed] [Google Scholar]
- 13.Hristov DR, Mahon E, Dawson KA. Chem Commun. 2015;51:17420–17423. doi: 10.1039/c5cc06598d. [DOI] [PubMed] [Google Scholar]
- 14.Malay O, Yilgor I, Menceloglu YZ. J Sol-Gel Sci Technol. 2013;67:351–361. [Google Scholar]
- 15.Dingsøyr E, Christy AA. In: Surface and Colloid Science. Razumas PV, Lindman PB, Nylander DT, editors. Springer; Berlin Heidelberg: 2000. pp. 67–73. [Google Scholar]
- 16.Thanh NTK, Maclean N, Mahiddine S. Chem Rev. 2014;114:7610–7630. doi: 10.1021/cr400544s. [DOI] [PubMed] [Google Scholar]
- 17.Stöber W, Fink A, Bohn E. J Colloid Interface Sci. 1968;26:62–69. [Google Scholar]
- 18.Carcouët CCMC, van de Put MWP, Mezari B, Magusin PCMM, Laven J, Bomans PHH, Friedrich H, Esteves ACC, Sommerdijk NAJM, van Benthem RATM, de With G. Nano Lett. 2014;14:1433–1438. doi: 10.1021/nl404550d. [DOI] [PubMed] [Google Scholar]
- 19.Davis TM, Snyder MA, Krohn JE, Tsapatsis M. Chem Mater. 2006;18:5814–5816. [Google Scholar]
- 20.Huang Y, Pemberton JE. Colloids Surf Physicochem Eng Asp. 2010;360:175–183. [Google Scholar]
- 21.Ding T, Yao L, Liu C. Nanoscale. 2016;8:4623–4627. doi: 10.1039/c5nr08224b. [DOI] [PubMed] [Google Scholar]
- 22.Yokoi T, Sakamoto Y, Terasaki O, Kubota Y, Okubo T, Tatsumi T. J Am Chem Soc. 2006;128:13664–13665. doi: 10.1021/ja065071y. [DOI] [PubMed] [Google Scholar]
- 23.Chen S-L, Dong P, Yang G-H, Yang J-J. Ind Eng Chem Res. 1996;35:4487–4493. [Google Scholar]
- 24.Bhakta S, Dixit CK, Bist I, Jalil KA, Suib SL, Rusling JF. Mater Res Express. 2016;3:075025. doi: 10.1088/2053-1591/3/7/075025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinker CJ, Scherer GW. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. 1. Academic Press; London, UK: 1990. pp. 97–228. [Google Scholar]
- 26.Xu Y, Sun X, Wu D, Sun Y, Yang Y, Yuan H, Deng F, Wu Z. J Solut Chem. 2007;36:327–344. [Google Scholar]
- 27.Rimer JD, Trofymluk O, Navrotsky A, Lobo RF, Vlachos DG. Chem Mater. 2007;19:4189–4197. [Google Scholar]
- 28.Tobler DJ, Shaw S, Benning LG. Geochim Cosmochim Acta. 2009;73:5377–5393. [Google Scholar]
- 29.Bailey JK, Mecartney ML. Colloids Surf. 1992;63:151–161. [Google Scholar]
- 30.Vreeland EC, Watt J, Schober GB, Hance BG, Austin MJ, Price AD, Fellows BD, Monson TC, Hudak NS, Maldonado-Camargo L, Bohorquez AC, Rinaldi C, Huber DL. Chem Mater. 2015;27:6059–6066. [Google Scholar]
- 31.Lovingood DD, Owens JR, Seeber M, Kornev KG, Luzinov I. ACS Appl Mater Interfaces. 2012;4:6875–6883. doi: 10.1021/am3020247. [DOI] [PubMed] [Google Scholar]
- 32.Costa CAR, Leite CAP, Galembeck F. J Phys Chem B. 2003;107:4747–4755. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


