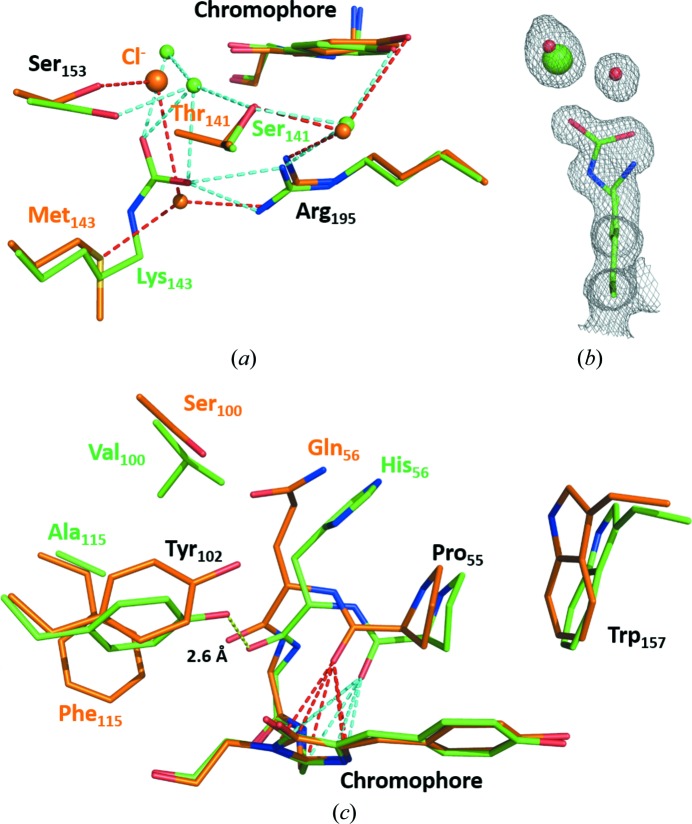
Figure 3.
Comparison of the environment of the chromophore in lanYFP (orange) and in mNeonGreen (green) at pH 8.0. (a) Close-up of the chloride-binding site in lanYFP and the carboxylated lysine in mNeonGreen at physiological pH. (b) σA-weighted 2F o − F c electron-density map contoured at a 1.0σ level around Lys143 in mNeonGreen, the major conformation of which is carboxylated and the minor conformation of which allows the binding of a chloride ion. (c) Close-up of the differences located on the other side of the chromophore. The strong hydrogen bond between Tyr102 and the carbonyl group of His56 in mNeonGreen is represented as a yellow dashed line. The lone pair–π interaction between the carbonyl group of Pro55 and the imidazolinone ring of the chromophore is represented as red dashed lines in lanYFP and cyan dashed lines in mNeonGreen