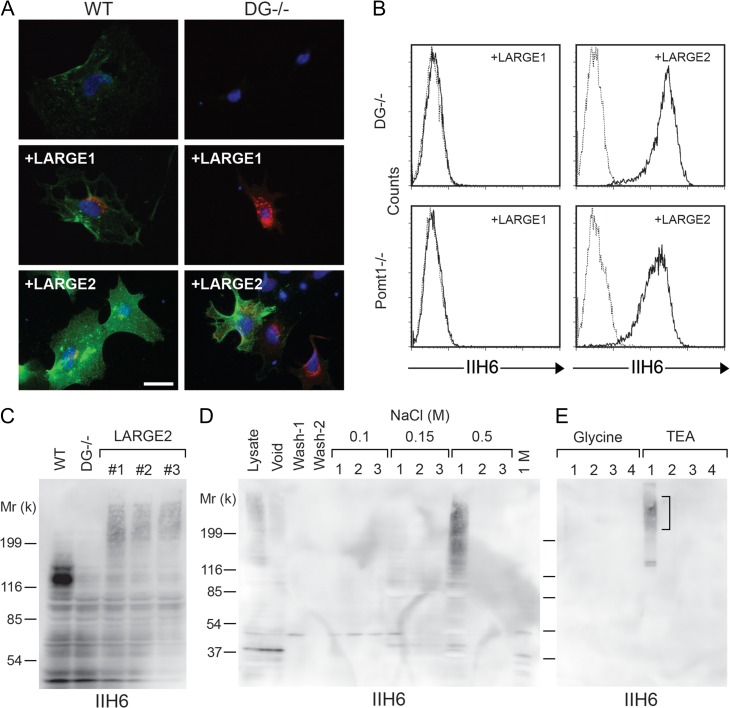
Fig. 1.
LARGE2 but not LARGE1 confers IIH6 immunoreactivity to DG−/− ES cells. (A) WT and DG−/− ES cells were infected with adenovirus to express LARGE1 or LARGE2. Cells were incubated with IIH6 (antibody that recognizes the functional glycan epitope of α-DG) and either anti-LARGE1 or anti-LARGE2 antibody, and then probed with Alexa-conjugated secondary antibodies (green pseudo-color: IIH6; red: LARGE1 or LARGE2; blue: DAPI). Scale bar, 50 μm. (B) Flow cytometry detecting surface IIH6 labeling of DG−/− cells or Pomt1−/− cells stably expressing either LARGE1 or LARGE2. Dashed line, secondary antibody alone. (C) Immunoblotting of whole cell lysate with IIH6. The WT sample shows a robust 120-kD band that corresponds to α-DG and is not present in DG−/− samples. Independent DG−/− cell clones stably expressing LARGE2 (#1–3) show a high-molecular weight smear that is absent in WT and the DG−/− cells. (D) IIH6 immunoblotting of eluted fractions from a diethylaminoethyl (DEAE)-cellulose column. Lysate of the DG−/− cells stably expressing LARGE2 was applied to the column. After two washes (Wash-1 and Wash-2), bound proteins were released by three sequential elutions with increasing concentrations of NaCl. (E) IIH6 immunoblotting of eluted fractions from a IIH6-Sepharose column. Bound proteins were eluted by four sequential glycine buffer washes, and then with triethanolamine (TEA) buffer. The high-molecular weight smear (square bracket) and two bands ~130-kD were excised from a SYPRO Ruby-stained gel, and were then subjected to in-gel trypsin digestion and mass spectrometric (MS) analysis (Table I). This figure is available in black and white in print and in color at Glycobiology online.