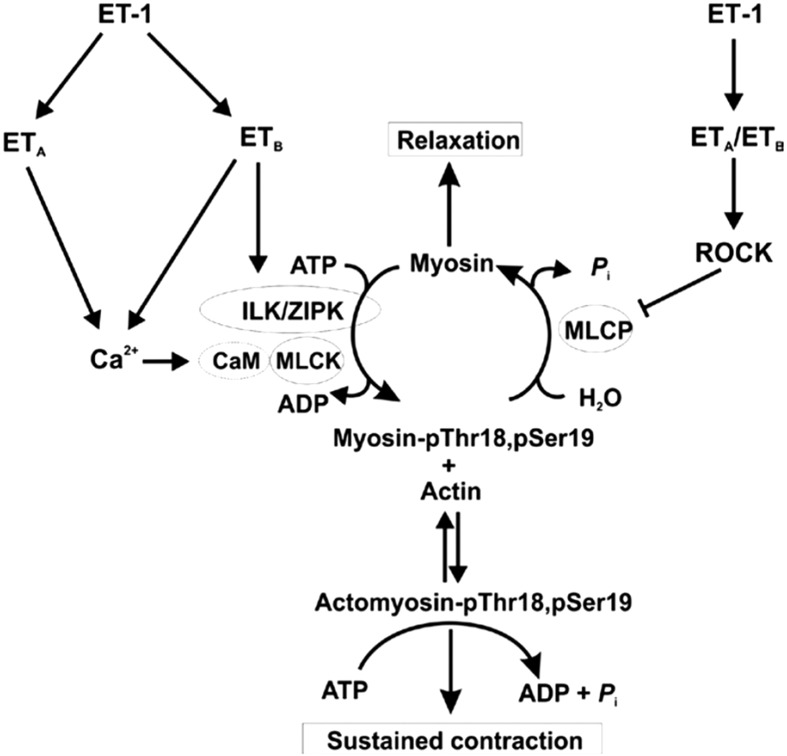
Fig. 5.
Proposed signaling pathways leading to ET-1-induced sustained vasoconstriction of the renal afferent arteriole. ET-1, acting via ETA receptors, triggers an increase in cytosolic free Ca2+ concentration ([Ca2+]i), largely through Ca2+ entry via voltage-gated Ca2+ channels (Cav), leading to binding of Ca2+ to calmodulin (CaM), activation of myosin light chain kinase (MLCK), phosphorylation of LC20 at Ser19 and cross-bridge cycling. Activation of ETA receptors also induces inhibition of myosin light chain phosphatase (MLCP) via activation of the Rho-associated kinase (ROCK) pathway, leading to increased LC20 phosphorylation at Ser19 due to the increase in MLCK: MLCP activity ratio. MLCP inhibition unmasks basal activity of integrin-linked kinase (ILK) and/or zipper-interacting protein kinase (ZIPK) that phosphorylate LC20 at both Thr18 and Ser19. Activation of ETB receptors in the vascular smooth muscle cells leads to activation of ILK and/or ZIPK (or possibly other kinase(s) capable of phosphorylating LC20 at Thr18 and Ser19). The rapid increase in Ser19 phosphorylation accounts for the initial phase of the contractile response to ET-1, while the slower diphosphorylation at Thr18 and Ser19, associated with reduced rates of LC20 dephosphorylation and relaxation (44), can account for the sustained contractile response to ET-1 and prolonged contraction that occurs following removal of the stimulus. This figure was originally published in Kidney International. Takeya K et al. Endothelin-1, but not angiotensin II, induces afferent arteriolar myosin diphosphorylation as a potential contributor to prolonged vasoconstriction. 2015; 87(2): 370–81. © International Society of Nephrology.