Abstract
Studies that evaluate the mechanisms for increased airway responsiveness are very sparse, although there are reports of exercise-induced bronchospasm. Therefore, we have evaluated the tracheal reactivity and the rate of lipid peroxidation after different intensities of swimming exercise in rats. Thus, male Wistar rats (age 8 weeks; 250–300 g) underwent a forced swimming exercise for 1 h whilst carrying attached loads of 3, 4, 5, 6 and 8% of their body weight (groups G3, G4, G5, G6 and G8, respectively; n=5 each). Immediately after the test, the trachea of each rat was removed and suspended in an organ bath to evaluate contractile and relaxant responses. The rate of lipid peroxidation was estimated by measuring malondialdehyde levels. According to a one-way ANOVA, all trained groups showed a significant decrease in the relaxation induced by aminophylline (10−12–10−1 M) (pD2=3.1, 3.2, 3.3, 3.3 and 3.2, respectively for G3, G4, G5, G6 and G8) compared to the control group (pD2=4.6) and the Emax values of G5, G6, G8 groups were reduced by 94.2, 88.0 and 77.0%, respectively. Additionally, all trained groups showed a significant increase in contraction induced by carbachol (10−9–10−3 M) (pD2=6.0, 6.5, 6.5, 7.2 and 7.3, respectively for G3, G4, G5, G6 and G8) compared to the control group (pD2=5.7). Lipid peroxidation levels of G3, G4 and G5 were similar in both the trachea and lung, however G6 and G8 presented an increased peroxidation in the trachea. In conclusion, a single bout of swimming exercise acutely altered tracheal responsiveness in an intensity-related manner and the elevation in lipid peroxidation indicates a degree of oxidative stress involvement.
Keywords: trachea, aerobic exercise, anaerobic exercise, lipid peroxidation
Introduction
Physical exercise is a primary indication for asthma treatment (1, 2), since it is known that training on asthmatics reduces levels of both leukotrienes and endothelin (3) as well as attenuating oxidative stress markers (4). Asthmatic exercisers have a lower bronchoconstriction, even though they were without a therapy to eliminate the hyperresponsiveness of the airways. Additionally, physical exercise improves aerobic capacity and the endurance of respiratory muscles, making asthma attacks less uncomfortable (2, 5).
On the other hand, acute physical exercise can promote a response known as exercise-induced bronchoconstriction (EIB) (6, 7), also called exercise-induced asthma. It is a transient bronchoconstriction that occurs during, or immediately after, vigorous exercise in some individuals. Its prevalence is 12–15% in the general population or 10–20% in Olympic athletes in summer conditions, however, this event increases to 50–70% of athletes under winter conditions and is intensified in patients with poorly controlled asthma (8).
Some investigations have identified that genetic (9), metabolic (10, 11) and hormonal factors (12) are related to EIB. Barreto et al. (11) showed that there is an increase in the concentration of 8-isoprostane (a marker of oxidative stress) in the breath condensate of asthmatic children with EIB, suggesting an involvement of oxidative stress in bronchial hyperactivity. In turn, other studies suggest that increased oxidative stress induces an increase in airway contractility (1).
The literature recommends that asthmatics should exercise with moderate intensity (13), but there is no data to support the concept that exercise intensity may be a causal factor in EIB. Several experimental models of tissue reactivity of the airways have been used to evaluate several factors such as the inflammatory processes involved in respiratory diseases (14) and the physiological mechanisms responsible for the production of mucus in the airways (15). Therefore, such models can provide information about a safe level of exercise which will avoid EIB in the population predisposed to this phenomenon. Despite this possibility, so far, there has been no study on the involvement of airway reactivity to exercise.
Thus, this study aimed to evaluate relaxation and contraction parameters in the rat trachea immediately after swimming exercise of different intensities, ranging from aerobic to anaerobic and to investigate the possible involvement of metabolites of lipid peroxidation produced during exercise.
Material and Methods
Animals
Male Wistar rats (age 8 wk; weight 250–300 g) were obtained from the bioterium of Prof. Thomas George in Centro de Biotecnologia (CBiotec)/Universidade Federal da Paraíba (UFPB). The animals were kept in appropriate cages and fed a balanced diet based on feed pellets (Labina®, Purina - Paulínia, Brasil) with water ad libitum, constant ventilation and temperature (21 ± 1 °C), and maintained in a daily 12-h light-dark cycle. The exercise tests were performed from 8–9 am and the cumulative concentration–response curves were constructed from 9 am – 10 pm. This study was performed following the guidelines for the ethical use of animals by Guide for Australian code for the care and use of animals for scientific purposes (2013). The protocol of this study was approved by the Ethics Committee for Animal Use of the CBiotec (CEUA/CBiotec) from UFPB (protocol: 1101/11).
Drugs
Calcium chloride dihydrate (CaCl2.2H2O), magnesium chloride hexahydrate (MgCl2.6H2O), potassium chloride (KCl) and sodium bicarbonate (NaHCO3) were purchased from VETEC (Brazil). Monosodium phosphate 1-hydrate (NaH2PO4.H2O), glucose (C6H12O6), magnesium sulfate monohydrate (MgSO4.H2O) and hydrochloric acid (HCl) were purchased from Nuclear (Brazil). Sodium chloride (NaCl) was purchased from Dinâmica (Brazil). Carbamylcholine chloride (CCh), aminophylline, trichloroacetic acid, arachidonic acid (AA), and thiobarbituric acid were purchased from Sigma-Aldrich (Brazil). Carbogenic mixture (95% O2 and 5% CO2) was purchased from White Martins (Brazil).
Exercise program
The animals were randomly divided into 6 groups with 5 rats in each. In the exercised groups, the animals were submitted to 1 h swimming sessions at five different exercise intensities (G3, G4, G5, G6 and G8%) (see below). In the Control Group (CG), the animals were maintained under the same conditions as the other groups and were acclimatized on the experiment day. They were not submitted to swimming session but to the control session where they were kept in shallow clean water at 31 ± 1 °C throughout the entire experimental period and were euthanized at the end of the experiment. The purpose of the control session was to induce stress without promoting physical training adaptations (16).
With stress, some animals had a tendency to adopt passive strategies termed immobilization periods, characterized by actions such as sitting on the bottom of the tank, putting the legs at the bottom of the tank to stand immobile, floating or carrying out small movements to keep their heads out of the water. To eliminate such stress, one week before the experimental protocol, the animals underwent two adaptive sessions, with an interval of 48 h between them. These sessions involved them swimming for 30 min without increasing the load they carried (17). The behavior of each animal was analyzed within the first 15 min, which was the duration recorded in the forced swimming test (18). Then, those animals that spent more than 15 min being immobile were excluded from our exercise trials, while the rats that swam actively were randomly distributed in each exercise group.
The swimming protocol was adapted from Chies et al. (19). We used a rectangular polyethylene tank measuring 120 cm in length, 50 cm in deep and 43 cm wide with water at a temperature of 29 ± 1 °C. The animals were submitted to the swimming exercise for a period of 1 h, with a metal ring tied to their chests by a 1-cm wide elastic ribbon. The ribbon was elastic and could be adjusted close to the chest of the animal, similar to a belt measure heart rate in humans. This was adjusted to prevent discomfort or stress, and in such a way that the movement of the animals was not restricted or that the load did not fall off during exercise. On the few occasions that the elastic ribbon slipped off the rat's chest, the researcher paused the timer, adjusting the ribbon and continued the exercise test. The metal rings in groups G3, G4, G5, G6 and G8 represented 3, 4, 5, 6 and 8% of the animal's body weight, respectively, corresponding to the exercise intensity range they were subjected to (19). Gobatto et al. (16) identified that in the rats the maximal lactate steady state level (MLSS) is achieved with exercise loads of 5 to 6%. Thus, in the present study we selected exercise intensities below the anaerobic threshold (3 and 4%), around the anaerobic threshold (5 and 6%), and finally one protocol above the threshold (8%).
Assessment of exercise-induced lactate
Immediately at the end of the exercise sessions, 25 µl of arterial blood was withdrawn from the tail artery of the animal (20) into calibrated heparinized capillaries. Shortly thereafter, the samples were placed in Eppendorf® tubes containing 400 µl of 4% trichloroacetic acid and refrigerated until analysis. For serum lactate levels the protocol proposed by Engel and Jones was followed (21).
Organ preparation and pharmacological evaluation of the rat trachea responsiveness
Five min after exercise, the rats of each group were euthanized by cervical dislocation. The trachea were removed and divided into segments containing 3–4 cartilage rings. To obtain isometric responses, the rings were individually suspended between an isometric force transducer and a stainless steel hook in organ baths (5 ml) containing Krebs solution (mM) (NaCl 118.0, KCl 4.6, MgSO4 5.7, KH2PO4 1.1, CaCl2 2.5, Glucose 11.0; NaHCO3 25.0), adjusted to pH 7.4 and maintained at 37 C. The preparations were stabilized for a period of 1 h under a resting tension of 1 g and gassed with a carbonic mixture (95% O2 + 5% CO2).
After this stabilization period, a tonic contraction was induced by 10−6 M CCh, a muscarinic agonist. The integrity of the tracheal epithelium was observed by addition of 10−4 M AA to the organ bath (22), the rings that relaxed equal to or higher than 50% were considered to have an intact epithelium, while those with a relaxation equal to or less than 10% was considered to be without epithelium. After washing, the tracheal preparations were rested for 30 min, then a second tonic contraction was induced by 10−6 M CCh and aminophylline (10−12–10−1 M), a phosphodiesterase (PDE) inhibitor (23) was added cumulatively to the organ bath to obtain a relaxation curve. For pharmacological evaluation of the contractile reactivity, a cumulative curve was obtained by the addition of increasing concentrations of CCh (10−9–10−3 M) to the organ bath. The tracheal responsiveness was evaluated by comparing pD2 (negative logarithm of molar concentration of an agonist that produces 50% of its maximal effect) and Emax (maximum effect) values for control and trained groups. The maximal contraction obtained in the CG was considered to be 100% and the effect exerted by the exercise was assessed referring to it.
Assessment of oxidative stress induced by exercise in trachea and lung
The rate of lipid peroxidation in trachea and lungs was determined by measuring the chromogenic product of the 2 thiobarbituric acid (TBA) reaction with malondialdehyde (MDA). The tissues were washed with cold saline to minimize the interference of hemoglobin with free radicals and to remove adhered blood. The tissues were weighed and homogenized with KCl 10%. Samples (250 µl) were removed and warmed in a water bath at 37 °C for 1 h, then 400 µl of perchloric acid 35% was added, and centrifuged at 0.02 G for 20 min at 4 °C. The supernatant was removed and placed in contact with 400 µl of thiobarbituric acid 0.6% and the mixture incubated at 95–100 °C for 1 h. After cooling the mixture, the absorbance of the supernatant was read at 532 nm. A standard curve was generated using 1,1,3,3-tetrametoxipropane. The results were expressed as nmol MDA/mg protein. Protein concentration was measured using the Bradford method (24). For the determination of MDA concentration in each tissue sample the values were replaced by the absorbance values of the MDA standard curve obtained from different concentrations of a standard solution as described by Simonato (25). The data were normalized by dry weight present in a given volume of the sample, where the absorbance values were divided by the weight in grams of the tissue.
Statistical analysis
Data were expressed as mean ± standard error of the mean (S.E.M.) and were tested for normality and homogeneity using the Shapiro-Wilk and Levine, respectively. Comparisons between groups were made using one-way ANOVA, with post hoc Bonferroni's test. Value of P<0.05 were considered to be significant. The values of pD2 and Emax were calculated by non-linear regression. All results were analyzed using GraphPad Prism version 5.01 (GraphPad Software Inc., San Diego CA, USA).
Results
Assessment of exercise-induced lactate
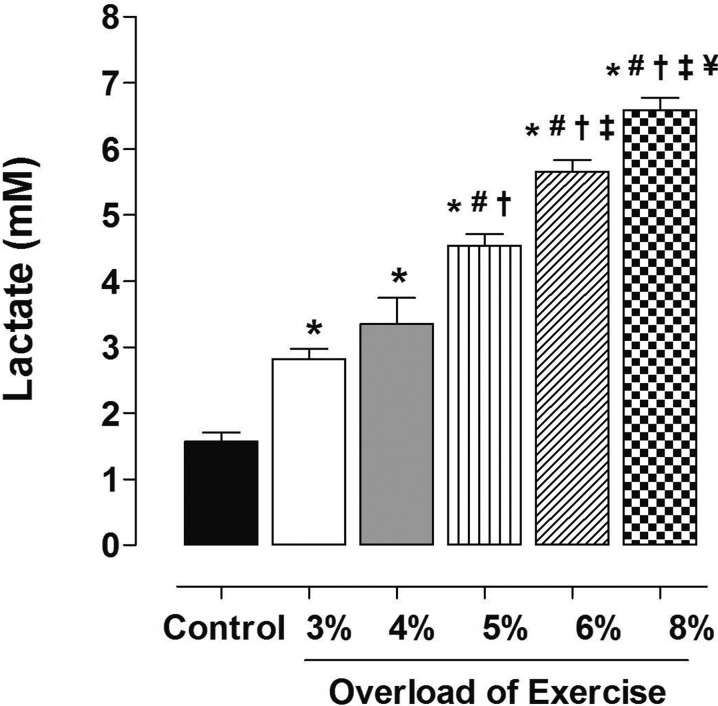
Figure 1 shows a significant increase in lactate content in response to exercise sessions, which was proportional to the intensity adopted. Between the intensities of 3% (G3, 2.8 ± 0.35 mM) and 4% (G4, 3.35 ± 0.80 mM) of animal body weight, there was no difference in lactate production. From this point forward, higher intensities resulted in lactate production significantly higher than the next lower intensity. Thus, 5% (G5, 4.54 ± 0.39 mM) promoted the production of lactate greater than 4%. The intensity of 6% (G6, 5.66 ± 0.39 mM) resulted in lactate production greater than 5% and the 8% intensity (G8, 6.59 ± 0.42 mM) resulted in lactacidemia greater than 6%.
Fig. 1.
Lactate production as a function of exercise intensity. Data are reported as means ± SEM (n = 5 per group). G3, G4, G5, G6 and G8: exercise intensity based on loading with 3, 4, 5, 6 and 8% of body weight, respectively, during swimming exercise. *P<0.05 vs. control; #P<0.05 vs. G3; †P<0.05 vs. G4; ‡P<0.05 vs. G5; ¥P<0.05 vs. G6. (one-way ANOVA test).
Pharmacological evaluation of the rat trachea responsiveness
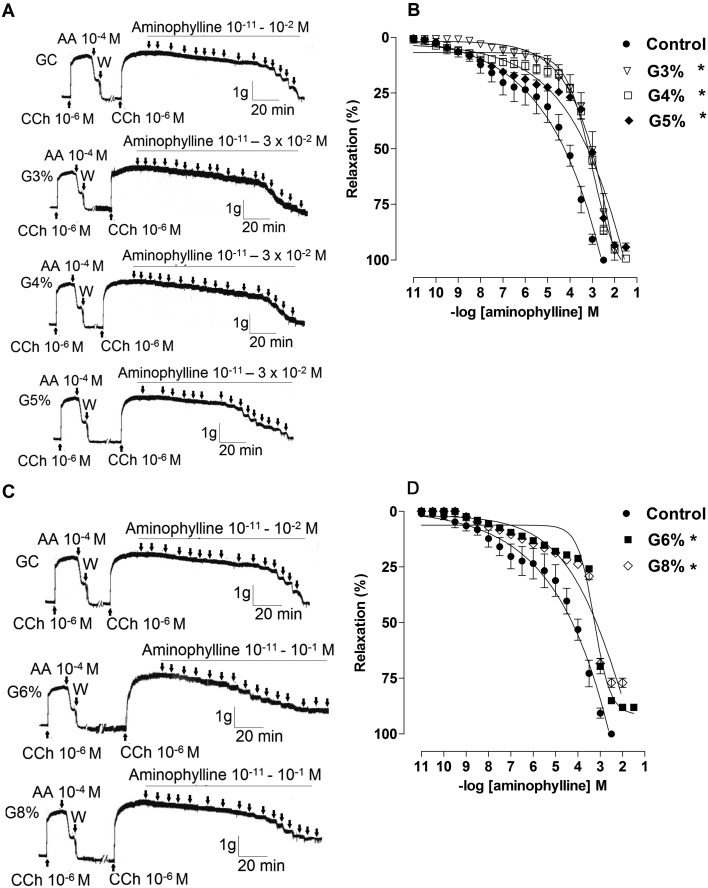
All exercised groups had a decrease in the percentage of relaxation at concentrations of aminophylline compared to CG (Table 1, Fig. 2), which is demonstrated by the values of pD2 to different intensities (G3, 3.1 ± 0.2; G4, 3.2 ± 0.05; G5, 3.3 ± 0.09; G6, 3.3 ± 0.02; G8, 3.2 ± 0.03 vs. CG, 4.6 ± 0.3, P<0.05). Furthermore, it was found that the values of Emax (%) in the G5, G6 and G8 were significantly reduced compared to CG, G3 and G4 (G5, 94.2 ± 1.77; G6, 88.0 ± 1.22; G8, 77.0 ± 2.09 vs. CG, 100.0 ± 0.0; G3, 99.60 ± 0.4; G4, 99.40 ± 0.6, P<0.05). It was also noted that Emax in G8 was lower than at 5% and 6% exercised groups (G8, 77.0 ± 2.09 vs. G5, 94.2 ± 1.77; G6, 88.0 ± 1.22, P<0.05).
Table 1. Values of pD2 and Emax (%) of aminophylline in the CG, G3, G4, G5, G6 and G8 groups on rat trachea.
| Group | Emax (%) | pD2 |
|---|---|---|
| CG | 100.0 ± 0.0 | 4.6 ± 0.3 |
| G3 | 99.60 ± 0.4 | 3.1 ± 0.2* |
| G4 | 99.40 ± 0.6 | 3.4 ± 0.05* |
| G5 | 94.2 ± 1.77*,# | 3.3 ± 0.09* |
| G6 | 88.0 ± 1.22*,# | 3.3 ± 0.02* |
| G8 | 77.0 ± 2.09*,#,¥ | 3.2 ± 0.03* |
One-way ANOVA followed by Bonferroni's post-test (n=5). G3, G4, G5, G6 and G8: exercise intensity based on loading with 3, 4, 5, 6 and 8% of body weight, respectively, during swimming exercise. *P<0.05 vs. control; #P<0.05 vs. G3 or G4; ¥P<0.05 vs. G5 or G6.
Fig. 2.
Representative traces and relaxant effect on rat trachea induced by aminophylline in control, G3, G4 and G5 (A and B, lower intensities), and G6 and G8 (C e D, higher intensities) groups. Data are reported as means ± SEM (n=5 per group). G3, G4, G5, G6 and G8: exercise intensity based on loading with 3, 4, 5, 6 and 8% of body weight, respectively, during swimming exercise. *P<0.05 vs. control (one-way ANOVA test). CCh: carbachol; AA: arachidonic acid; W: wash.
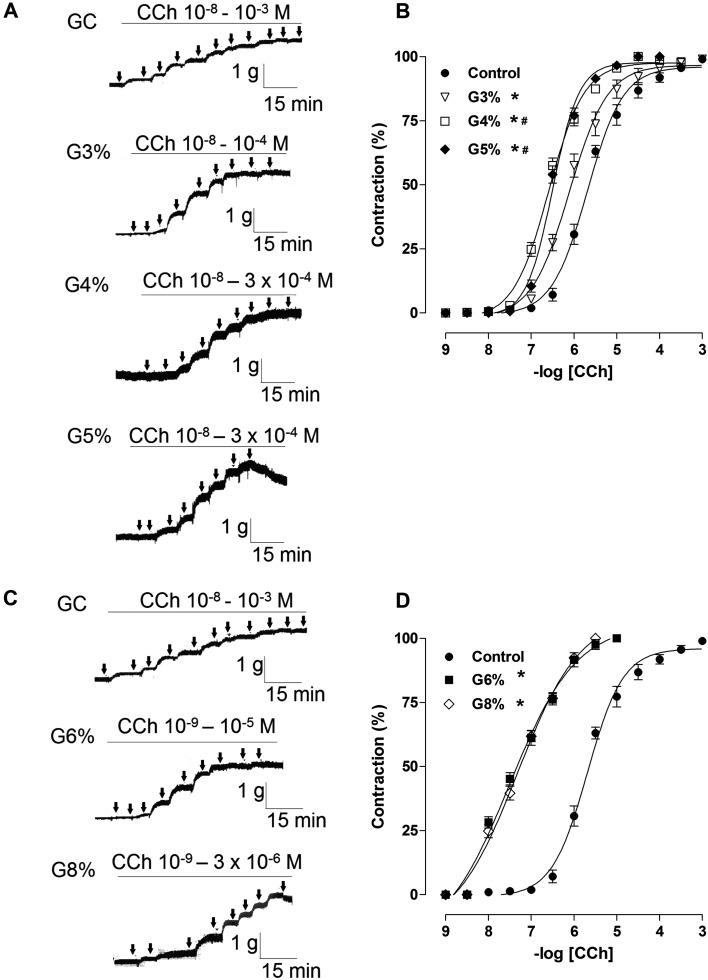
In addition, the exercised-induced groups showed a significant increase in their percentage of contraction in concentrations of CCh compared to the CG (Table 2, Fig. 3). The contractile response was significantly greater for G4 and G5 compared to G3 (Fig. 3A and B). However, the higher intensities of the exercised groups (G6 and G8) showed an overlap for its percentage of contraction when compared to other groups (Fig. 3C and D), as demonstrated by the pD2 values (G6, 7.2 ± 0.04; G8, 7.3 ± 0.05 vs. CG, 5.7 ± 0.06; G3, 6.0 ± 0.09; G4, 6.5 ± 0.02; G5, 6.5 ± 0.02, P<0.05).
Table 2. Values of pD2 of CCh in the CG, G3, G4, G5, G6 and G8 groups on rat trachea.
| Group | pD2 |
|---|---|
| CG | 5.7 ± 0.06 |
| G3 | 6.0 ± 0.09*,¥ |
| G4 | 6.5 ± 0.02*,#,¥ |
| G5 | 6.2 ± 0.02*,#,¥ |
| G6 | 7.2 ± 0.04* |
| G8 | 7.3 ± 0.05* |
One-way ANOVA followed by Bonferroni's post-test (n=5). G3, G4, G5, G6 and G8: exercise intensity based on loading with 3, 4, 5, 6 and 8% of body weight, respectively, during swimming exercise. *P<0.05 vs. control; #P<0.05 vs. G3 or G4; ¥P <0.05 vs. G6 or G8.
Fig. 3.
Representative traces and contractile effect on rat trachea induced by CCh in control, G3, G4 and G5 (A and B, lower intensities), and G6 and G8 (C e D, higher intensities) groups. Data are reported as the means ± SEM (n = 5 per group). G3, G4, G5, G6 and G8: exercise intensity based on loading with 3, 4, 5, 6 and 8% of body weight, respectively, during swimming exercise. *P<0.05 vs. control; #P<0.05 vs. G3 (one-way ANOVA test). CCh: carbachol.
Assessment of oxidative stress induced by exercise in trachea and lung
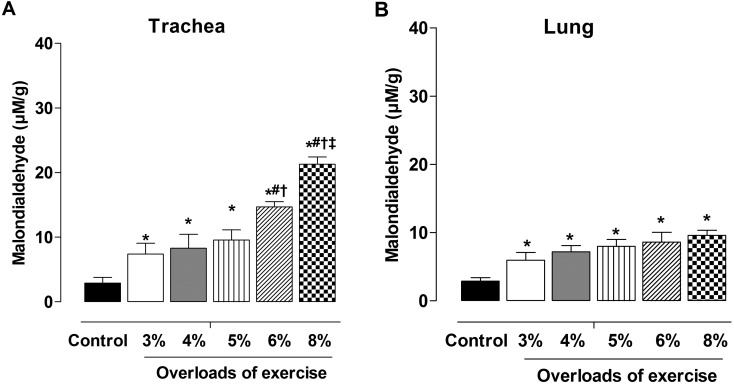
All exercise protocols promoted increases in lipid peroxidation unrelated to exercise intensity compared to the CG (Fig. 4). In rat trachea, the increase in peroxidation was similar among intensities of 3, 4 and 5%, while 6% presented much higher peroxidation and G8% generated the highest lipid peroxidation among all intensities. On lung samples, there was increased peroxidation but without differences among the intensities of exercises.
Fig. 4.
Levels of lipid peroxidation in trachea (A) and lung (B) in control, G3, G4, G5, G6 and G8 groups. Data are reported as means ± SEM (n=5 per group). G3, G4, G5, G6 and G8: exercise intensity based on loading with 3, 4, 5, 6 and 8% of body weight, respectively, during swimming exercise. *P< 0.05 vs. control; #P<0.05 vs. G4; †P<0.05 vs. G5; ‡P<0.05 vs. G6 (one-way ANOVA test).
Discussion
The present study demonstrated that aerobic exercise promotes a reduction in the tracheal relaxation response accompanied by an increased contractile pattern of this organ which was exacerbated at higher intensities (6 and 8%). The contraction increased and the relaxation reduced, with loads of 6 and 8 and were directly related with a significant elevation in lipid peroxidation (rat trachea and lungs). There are corroborating studies that showed that oxidative stress can induce airway hyperresponsiveness (26), mucus hypersecretion (27), epithelial shedding (28) and produce harmful effects on the airway smooth muscle (29).
Asthmatic disease is a chronic inflammatory pathology characterized by hyperresponsiveness of the lower airways to several allergenic factors, which contracts the bronchioles hindering expiration severely (30). On the other hand, EIB or exercise-induce asthma may occur even in persons who do not respond to any allergen. The practical implication of the present data is associated with EIB.
It is known that interleukins and other pro-inflammatory agents (9), hormonal changes with increased levels of leptin and reduced adiponectin levels (12) and increased oxidative stress (11) may participate in EIB. In addition, environmental changes such as cold weather, dry air and even intensity of exercise are factors that can influence the bronchospasm (31). Despite these factors, the exercise intensity has been given special attention, because it is the variable most likely to be controlled in order to prevent EIB episodes (32). In light of this, the consensus is that moderate-intensity exercise is more suitable to achieve the benefits of physical training while minimizing asthma attacks in humans (33). In fact, some studies have shown a severe bronchospasm in response to high intensity exercise both in people with respiratory problems (34) and athletes (35, 36).
The mechanisms involved in this phenomenon include an increase in pro-inflammatory mediators (kinins and interleukins) (37), oxidant lipid mediators (38) and nitric oxide (39). It is noteworthy these mechanisms were mostly studied using only indirect methods such as questionnaires and measurement of gases. Studies that directly prove these mechanisms are sparse, but there is one investigation analyzing the expression of pro-inflammatory proteins in the adrenal medulla (32). Exercise has been shown to increase the expression of phosphorylated inflammatory proteins and consequently the levels of eosinophils in high-intensity exercises.
The present study provided direct data on the responsiveness (contractile and relaxant) of exercise on superior airways in an animal model. Our results showed that a range of low to moderate intensity aerobic exercises are able to increase the contractile response to cholinergic stimulation and reduced the relaxant patterns of the xanthine compound in Wistar rat trachea, and that higher intensity of exercise exacerbates these responses. In this study, we have used aminophylline instead of isoproterenol which stimulates the adrenoceptors because the rat tracheal smooth muscle has few β-receptors (40) and these receptors can be easily desensitized by prolonged or repetitive administration of isoproterenol. Although isoproterenol-desensitized tracheal preparations exhibited a diminished sensitivity to other β-agonists, they still respond to the spasmolytic actions of aminophylline (41). The intensities that modify the contractile and relaxant responsiveness were of 6 and 8% of body weight, which induced a metabolic demand of 5.66 and 6.59 mM of lactate. Therefore, we determinate that the bronchospasm was achieved in the anaerobic exercises range (16). The animals in this study were able to maintain the exercise protocol for 60 min with the same serum concentration of 5.5 mM, which was the lactate threshold as proposed by Gobatto et al. (16). In contrast, Carr et al. (42) found an anaerobic threshold of 7 mM, which explains in a better way the performance of the rats in our exercise protocol. Therefore, our results allow us to propose that higher intensity exercise, but not necessarily anaerobic, would be less suitable for those who want to avoid EIB attacks.
Hewitt et al. (43) indicate that even moderate-intensity aerobic exercise training attenuates the airway hyperresponsiveness via a mechanism that involves β2-adrenoceptors in mice sensitized/challenged with ovalbumin, but not in the control group. Furthermore, low-intensity aerobic exercise has been associated with an increase in the levels of serum epinephrine mitigating EIB in asthmatic Sprague-Dawley rats (44). In these studies, the exercise attenuated the airway resistance in asthmatic rats, but not in normal rats, however, the species used in our study differs from theirs.
In addition to the increased contractile response in higher intense workouts, the elevation in lipid peroxidation, as a function of exercise intensity, indicates that oxidative stress may be also involved in the inflammatory activity of airway, as it has been described that reactive oxygen species can induce cytokine and chemokine production through induction of the oxidative stress-sensitive transcription of nuclear factor-kB in bronchial epithelial cells (45).
These data corroborate what was previously suggested by Barreto et al. (11), who reported an increase in the concentration of 8-isoprostane (an indicator of oxidative stress) in the exhalation of children with exercise-induced bronchoconstriction. Other recent studies also found an increased oxidative stress in humans who did exercise with higher intensities (3, 38).
In the present experiments we applied to the animal models a training protocol which was similar to that for humans and observed, for the first time, its acute influences on superior airway responsiveness. The results suggest that the airways become more responsive to contractile agents and less to relaxant agents in response to exercise with high intensity, allowing us to provide more substantial data to support the idea that the asthmatics should choose the lower intensity exercises from light to moderate. The incidence of asthma exercise-induced is intensity-dependent and the threshold intensity may be reduced in the case of humans or animals, having a history of asthma. Therefore, further experiments using asthmatic animal models should be done to characterize our findings better. Finally, the present study is of great value for both the patients and the exercise prescribers since it provides a guiding principle for the physical activities that will be given to patients with respiratory problems.
Conflict of interest
The authors declare that they have no conflict of interest.
Authors' contributions
AFB, ASS and BAS designed the study. AFB, ILLS and JCP performed the study. AFB, ILLS and IRRM performed data analysis and drafted the manuscript.
Acknowledgments
The authors thank CAPES, CNPq and FAPESQ-PB for their financial support and UFPB for structural support.
References
- 1.Lucas SR, Platts-Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol. 2005; 115(5): 928–34. doi: 10.1016/j.jaci.2005.01.033 [DOI] [PubMed] [Google Scholar]
- 2.Morton AR, Fitch KD. Australian association for exercise and sports science position statement on exercise and asthma. J Sci Med Sport. 2011; 14(4): 312–6. doi: 10.1016/j.jsams.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 3.Gunay O, Onur E, Yilmaz O, Dundar PE, Tikiz C, Var A, Yuksel H. Effects of physical exercise on lung injury and oxidant stress in children with asthma. Allergol Immunopathol (Madr). 2012; 40(1): 20–4. doi: 10.1016/j.aller.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 4.Onur E, Kabaroğlu C, Günay O, Var A, Yilmaz O, Dündar P, Tikiz C, Güvenç Y, Yüksel H. The beneficial effects of physical exercise on antioxidant status in asthmatic children. Allergol Immunopathol (Madr). 2011; 39(2): 90–5. doi: 10.1016/j.aller.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 5.Mendes FA, Lunardi AC, Silva RA, Cukier A, Stelmach R, Martins MA, Carvalho CR. Association between maximal aerobic capacity and psychosocial factors in adults with moderate-to-severe asthma. J Asthma. 2013; 50(6): 595–9. doi: 10.3109/02770903.2013.786724 [DOI] [PubMed] [Google Scholar]
- 6.Anderson SD, Brannan JD. Long-acting beta 2-adrenoceptor agonists and exercise-induced asthma: lessons to guide us in the future. Paediatr Drugs. 2004; 6(3): 161–75. doi: 10.2165/00148581-200406030-00003 [DOI] [PubMed] [Google Scholar]
- 7.Dryden DM, SPooner CH, STickland MK, Vandermeer B, Tjosvold L, Bialy L, Wong K, Rowe BH. Exercise-induced bronchoconstriction and asthma. Evid Rep Technol Assess. 2010; 189: 1–154. [PMC free article] [PubMed] [Google Scholar]
- 8.Hildebrand K. [Exercise-induced bronchoconstriction]. Pneumonol Alergol Pol. 2011; 79(1): 39–47. [PubMed] [Google Scholar]
- 9.Kang MJ, Lee SY, Kim HB, Yu J, Kim BJ, Choi WA, Jang SO, Hong SJ. Association of IL-13 polymorphisms with leukotriene receptor antagonist drug responsiveness in Korean children with exercise-induced bronchoconstriction. Pharmacogenet Genomics. 2008; 18(7): 551–8. doi: 10.1097/FPC.0b013e3282fe94c5 [DOI] [PubMed] [Google Scholar]
- 10.Zietkowski Z, Skiepko R, Tomasiak MM, Bodzenta-Lukaszyk A. Soluble CD40 ligand and soluble P-selectin in allergic asthma patients during exercise-induced bronchoconstriction. J Investig Allergol Clin Immunol. 2008; 18(4): 272–8. [PubMed] [Google Scholar]
- 11.Barreto M, Villa MP, Olita C, Martella S, Ciabattoni G, Montuschi P. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest. 2009; 135(1): 66–73. doi: 10.1378/chest.08-0722 [DOI] [PubMed] [Google Scholar]
- 12.Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011; 107(1): 14–21. doi: 10.1016/j.anai.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 13.Apter AJ. Advances in adult asthma diagnosis and treatment in 2009. J Allergy Clin Immunol. 2010; 125(1): 79–84. doi: 10.1016/j.jaci.2009.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokry J, Joskova M, Mokra D, Christensen I, Nosalova G. Effects of selective inhibition of PDE4 and PDE7 on airway reactivity and cough in healthy and ovalbumin-sensitized guinea pigs. Adv Exp Med Biol. 2013; 756: 57–64. doi: 10.1007/978-94-007-4549-0_8 [DOI] [PubMed] [Google Scholar]
- 15.Mazzone SB, Lim LH, Wagner EM, Mori N, Canning BJ. Sympathetic nerve-dependent regulation of mucosal vascular tone modifies airway smooth muscle reactivity. J Appl Physiol 1985. 2010; 109(5): 1292–300. doi: 10.1152/japplphysiol.00632.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobatto CA, de Mello MA, Sibuya CY, de Azevedo JR, dos Santos LA, Kokubun E. Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A Mol Integr Physiol. 2001; 130(1): 21–7. doi: 10.1016/S1095-6433(01)00362-2 [DOI] [PubMed] [Google Scholar]
- 17.Bruner CA, Vargas I. The activity of rats in a swimming situation as a function of water temperature. Physiol Behav. 1994; 55(1): 21–8. doi: 10.1016/0031-9384(94)90004-3 [DOI] [PubMed] [Google Scholar]
- 18.Martí J, Armario A. Effects of diazepam and desipramine in the forced swimming test: influence of previous experience with the situation. Eur J Pharmacol. 1993; 236(2): 295–9. doi: 10.1016/0014-2999(93)90601-D [DOI] [PubMed] [Google Scholar]
- 19.Chies AB, de Oliveira AM, Pereira FC, de Andrade CR, Corrêa FM. Phenylephrine-induced vasoconstriction of the rat superior mesenteric artery is decreased after repeated swimming. J Smooth Muscle Res. 2004; 40(6): 249–58. doi: 10.1540/jsmr.40.249 [DOI] [PubMed] [Google Scholar]
- 20.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010; 1(2): 87–93. doi: 10.4103/0976-500X.72350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engel PC, Jones JB. Causes and elimination of erratic blanks in enzymatic metabolite assays involving the use of NAD+ in alkaline hydrazine buffers: improved conditions for the assay of L-glutamate, L-lactate, and other metabolites. Anal Biochem. 1978; 88(2): 475–84. doi: 10.1016/0003-2697(78)90447-5 [DOI] [PubMed] [Google Scholar]
- 22.Tschirhart E, Frossard N, Bertrand C, Landry Y. Arachidonic acid metabolites and airway epithelium-dependent relaxant factor. J Pharmacol Exp Ther. 1987; 243(1): 310–6. [PubMed] [Google Scholar]
- 23.Matsuda F, Sugahara K, Sugita M, Sadohara T, Kiyota T, Terasaki H. Comparative effect of amrinone, aminophylline and diltiazem on rat airway smooth muscle. Acta Anaesthesiol Scand. 2000; 44(6): 763–6. doi: 10.1034/j.1399-6576.2000.440617.x [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248–54. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 25.Simonato A. Efeitos do peróxido de hidrogênio (H2O2) sobre os mecanismos de transdução de sinal da camada longitudial de íleo de cobaia [dissertation]. São Paulo (SP): Universidade Federal de São Paulo/Escola Paulista de Medicina. 2002.
- 26.Katsumata U, Miura M, Ichinose M, Kimura K, Takahashi T, Inoue H, Takishima T. Oxygen radicals produce airway constriction and hyperresponsiveness in anesthetized cats. Am Rev Respir Dis. 1990; 141(5 Pt 1): 1158–61. doi: 10.1164/ajrccm/141.5_Pt_1.1158 [DOI] [PubMed] [Google Scholar]
- 27.Adler KB, Holden-Stauffer WJ, Repine JE. Oxygen metabolites stimulate release of high-molecular-weight glycoconjugates by cell and organ cultures of rodent respiratory epithelium via an arachidonic acid-dependent mechanism. J Clin Invest. 1990; 85(1): 75–85. doi: 10.1172/JCI114436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doelman CJA, Leurs R, Oosterom WC, Bast A. Mineral dust exposure and free radical-mediated lung damage. Exp Lung Res. 1990; 16(1): 41–55. doi: 10.3109/01902149009064698 [DOI] [PubMed] [Google Scholar]
- 29.Rhoden KJ, Barnes PJ. Effect of hydrogen peroxide on guinea-pig tracheal smooth muscle in vitro: role of cyclo-oxygenase and airway epithelium. Br J Pharmacol. 1989; 98(1): 325–30. doi: 10.1111/j.1476-5381.1989.tb16898.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vargas Becerra MH. [Physiopathology of asthma]. Rev Alerg Mex. 2009; 56(Suppl 1): S24–8. [PubMed] [Google Scholar]
- 31.Ali Z, Norsk P, Ulrik CS. Mechanisms and management of exercise-induced asthma in elite athletes. J Asthma. 2012; 49(5): 480–6. doi: 10.3109/02770903.2012.676123 [DOI] [PubMed] [Google Scholar]
- 32.He R, Feng J, Xun Q, Qin Q, Hu C. High-intensity training induces EIB in rats through neuron transdifferentiation of adrenal medulla chromaffin cells. Am J Physiol Lung Cell Mol Physiol. 2013; 304(9): L602–12. doi: 10.1152/ajplung.00406.2012 [DOI] [PubMed] [Google Scholar]
- 33.Weiler JM, Anderson SD, Randolph C, Bonini S, Craig TJ, Pearlman DS, Rundell KW, Silvers WS, Storms WW, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Schuller DE, Spector SL, Tilles SA, Wallace D, Henderson W, Schwartz L, Kaufman D, Nsouli T, Shieken L, Rosario N. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010; 105(6 Suppl): S1–47. doi: 10.1016/j.anai.2010.09.021 [DOI] [PubMed] [Google Scholar]
- 34.Baek HS, Cho J, Kim JH, Oh JW, Lee HB. Ratio of leukotriene e(4) to exhaled nitric oxide and the therapeutic response in children with exercise-induced bronchoconstriction. Allergy Asthma Immunol Res. 2013; 5(1): 26–33. doi: 10.4168/aair.2013.5.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansley L, Kippelen P, Dickinson J, Hull JH. Misdiagnosis of exercise-induced bronchoconstriction in professional soccer players. Allergy. 2012; 67(3): 390–5. doi: 10.1111/j.1398-9995.2011.02762.x [DOI] [PubMed] [Google Scholar]
- 36.Teixeira RN, Teixeira LR, Costa LA, Martins MA, Mickleborough TD, Carvalho CR. Exercise-induced bronchoconstriction in elite long-distance runners in Brazil. J Bras Pneumol. 2012; 38(3): 292–8. doi: 10.1590/S1806-37132012000300003 [DOI] [PubMed] [Google Scholar]
- 37.Li F, Zhang M, Hussain F, Triantaphyllopoulos K, Clark AR, Bhavsar PK, Zhou X, Chung KF. Inhibition of p38 MAPK-dependent bronchial contraction after ozone by corticosteroids. Eur Respir J. 2011; 37(4): 933–42. doi: 10.1183/09031936.00021110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Díaz-Castro J, Guisado R, Kajarabille N, García C, Guisado IM, De Teresa C, Ochoa JJ. Phlebodium decumanum is a natural supplement that ameliorates the oxidative stress and inflammatory signalling induced by strenuous exercise in adult humans. Eur J Appl Physiol. 2012; 112(8): 3119–28. doi: 10.1007/s00421-011-2295-3 [DOI] [PubMed] [Google Scholar]
- 39.Grzelewski T, Grzelewska A, Majak P, Stelmach W, Kowalska A, Stelmach R, Janas A, Stelmach I. Fractional exhaled nitric oxide (FeNO) may predict exercise-induced bronchoconstriction (EIB) in schoolchildren with atopic asthma. Nitric Oxide. 2012; 27(2): 82–7. doi: 10.1016/j.niox.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 40.O’Donnell SR, Wanstall JC, Mustafa MBH. Influence of thyroid status on responses of rat isolated pulmonary artery, vas deferens and trachea to smooth muscle relaxant drugs. Br J Pharmacol. 1987; 92(1): 221–9. doi: 10.1111/j.1476-5381.1987.tb11315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin CS, Hurwitz L, Jenne J, Avner BP. Mechanism of isoproterenol-induced desensitization of tracheal smooth muscle. J Pharmacol Exp Ther. 1977; 203(1): 12–22. [PubMed] [Google Scholar]
- 42.Carr AJ, Hopkins WG, Gore CJ. Effects of acute alkalosis and acidosis on performance: a meta-analysis. Sports Med. 2011; 41(10): 801–14. doi: 10.2165/11591440-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 43.Hewitt M, Estell K, Davis IC, Schwiebert LM. Repeated bouts of moderate-intensity aerobic exercise reduce airway reactivity in a murine asthma model. Am J Respir Cell Mol Biol. 2010; 42(2): 243–9. doi: 10.1165/rcmb.2009-0038OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin Q, Feng J, Hu C, Chen X, Qin L, Li Y. Low-intensity aerobic exercise mitigates exercise-induced bronchoconstriction by improving the function of adrenal medullary chromaffin cells in asthmatic rats. Tohoku J Exp Med. 2014; 234(2): 99–110. doi: 10.1620/tjem.234.99 [DOI] [PubMed] [Google Scholar]
- 45.Biagioli MC, Kaul P, Singh I, Turner RB. The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic Biol Med. 1999; 26(3-4): 454–62. doi: 10.1016/S0891-5849(98)00233-0 [DOI] [PubMed] [Google Scholar]