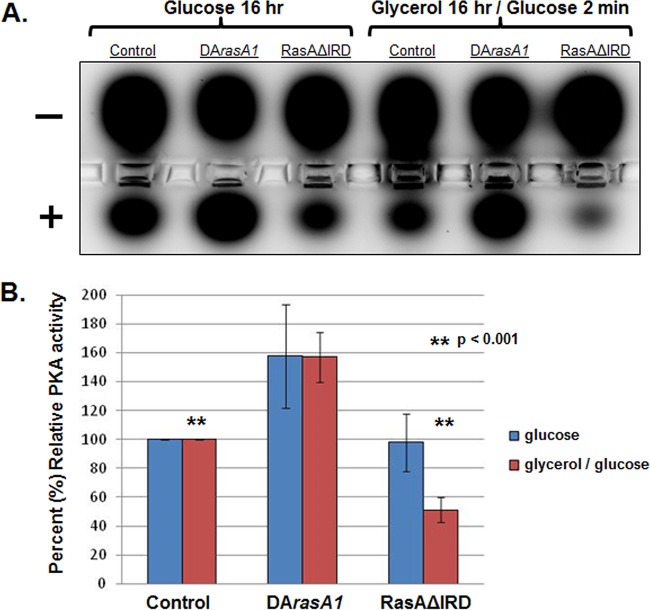
FIG 6 .
PKA activation is deficient in the RasAΔIRD mutant. (A) PKA activity assay performed on lysates of the control strain, a strain expressing constitutively active RasA (DArasA1), and the RasAΔIRD mutant. Equal numbers of conidia from each strain were cultured in either (i) AMM glucose-based medium (Glucose 16 hr) overnight or (ii) AMM modified to contain glycerol as the sole carbon source, followed by a 2-min stimulation with glucose (Glycerol 16 hr/Glucose 2 min). Lysates were generated from each strain and condition and assayed for PKA activity using the PepTag nonradioactive cAMP-dependent protein kinase assay (Promega). The agarose gel electrophoresis image is representative of three independent experiments and shows migration of phosphorylated PKA-target peptide toward the cathode (+) and unphosphorylated target toward the anode (−). Control experiments, including addition of the bovine PKA catalytic subunit and exogenous cAMP to measure induction of PKA activity, are provided in Fig. S3 in the supplemental material. (B) Densitometric quantification of phosphorylated PKA-target peptide (migrating toward the cathode) for each strain, relative to the control strain. Results are the average from three separate experiments ± standard deviation. Data were compared using Student’s t test.