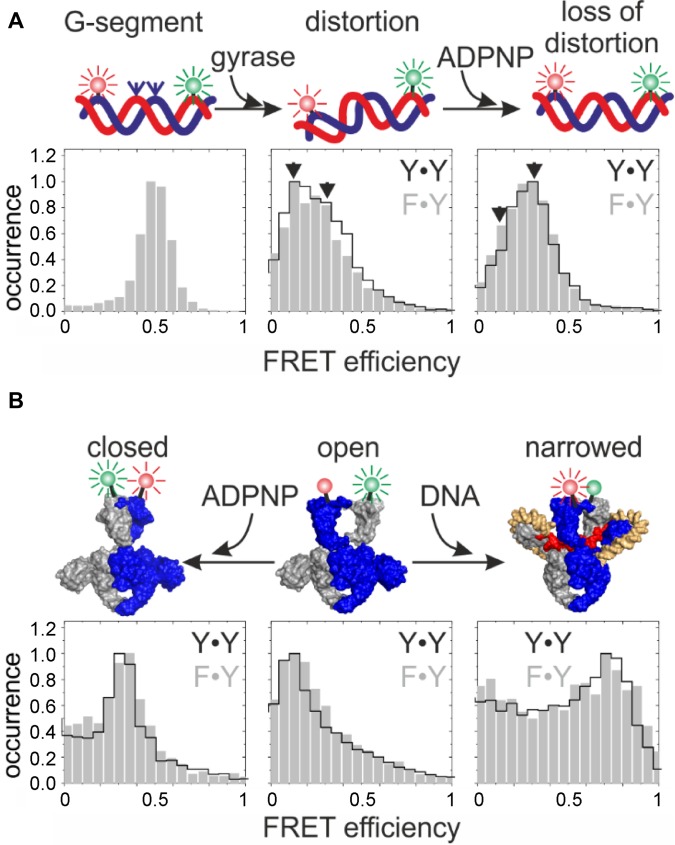
Figure 4.
Conformational changes at the beginning of the catalytic cycle are independent of the number of tyrosines present. (A) Conformational changes of the DNA, probed by smFRET with a 60 bp DNA that contains a preferred cleavage site for B. subtilis gyrase (49), flanked by donor and acceptor fluorophores (green and red spheres). smFRET histograms of DNA in the presence of gyrase containing two catalytic tyrosines (A2B2) are outlined in black, histograms in the presence of gyrase containing one tyrosine (B·AF·BA) are shown in gray. Left: DNA only; center: DNA distortion upon binding to gyrase, right: loss of distortion upon ADPNP binding. Gyrase induces identical DNA conformations, irrespective of the number of tyrosines present. (B) DNA- and nucleotide-induced conformational changes of gyrase. N-gate conformation was probed in smFRET experiments using BAF·BA (one tyrosine) and (BA)2 (two tyrosines), carrying a S7C mutation for fluorescent labeling (31). FRET histograms show identical conformations for the proteins in the absence of DNA or nucleotide (center), and DNA-induced N-gate narrowing (right) and nucleotide-induced N-gate closure (left) independent of the number of tyrosines present.