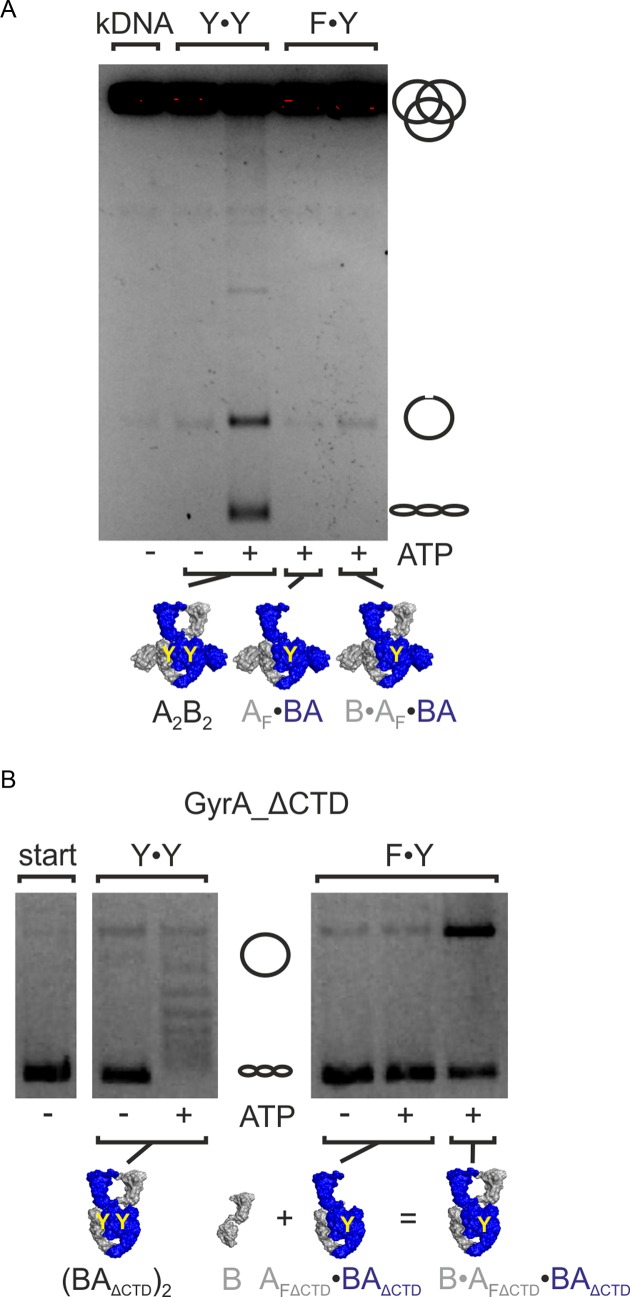
Figure 6.
ATP-dependent DNA relaxation and decatenation. (A) ATP-dependent decatenation of kDNA by gyrase. Gyrase with two catalytic tyrosines (Y·Y, A2B2) catalyzes decatenation in the presence of ATP, but not in its absence. Gyrase containing only one catalytic tyrosine (B·AF·BA) shows no or very little decatenation activity. The AF·BA heterodimer control shows no significant background activity. Thus, decatenation requires two catalytic tyrosines and strand passage. (B) ATP-dependent relaxation by gyrase lacking the CTDs. Gyrase lacking the CTDs and containing one catalytic tyrosine (F·Y; B·AFΔCTD·BAΔCTD) shows no ATP-dependent DNA relaxation activity. Gyrase lacking the CTDs but containing two catalytic tyrosines (Y·Y, (BAΔCTD)2) serves as a positive control. Concentrations were 500 nM AF·BA and 1 μM B, or 500 nM (BAΔCTD)2. kDNA: catenated DNA; start: DNA substrate; A2B2: heterotetrameric gyrase; AF: cleavage-deficient GyrA subunit (GyrAY123F), AFΔCTD: cleavage-deficient GyrA carrying the Y123F mutation and lacking the C-terminal domain; B: GyrB; BA: GyrBA-fusion protein; BAΔCTD: GyrBA lacking the CTDs.