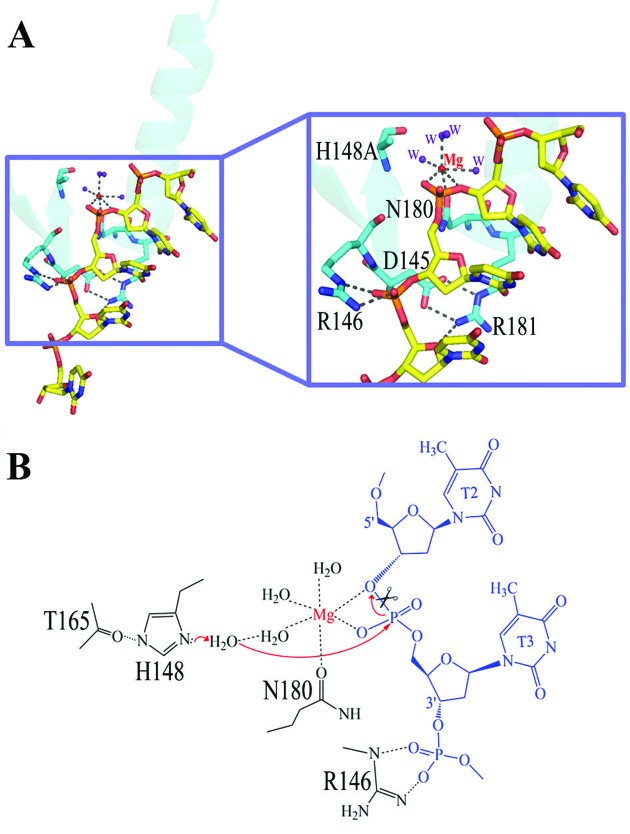
Figure 4.
The DNA hydrolysis mechanism by EndoG/CPS-6. (A) Close-up of the ββα-metal motifs of CPS-6 bound to DNA. (B) Schematic diagram of the proposed DNA hydrolysis mechanism by CPS-6. The Mg2+ located within the ββα-metal motif is coordinated by six ligands in an octahedral geometry: Asn180, two scissile phosphate oxygens, and three water molecules. Asp145 forms two salt bridges with the side chain of Arg181 to stabilize the conformation of the ββα-metal motif. His148 was mutated to Ala in the crystal structure and its side-chain is likely polarized by Thr165. His148 acts as a general base to activate a water molecule, which in turn makes an in-line attack on the scissile phosphate. The Mg-bound water functions as the general acid to provide a proton to the cleaved DNA product.