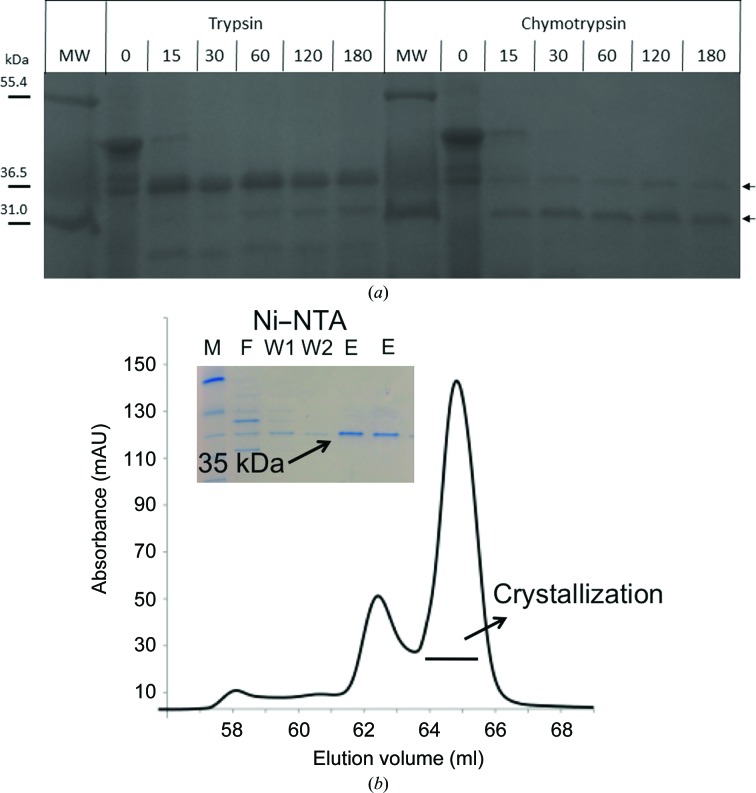
Figure 1.
(a) Limited proteolysis of FapF. SDS–PAGE gel showing samples of FapF digested over a range of time periods with trypsin and chymotrypsin, with samples taken periodically over the course of 180 min as indicated (labelled in minutes). The protein is processed to produce stable fragments of approximately 35 or 31 kDa (indicated with arrows). Lane MW contains molecular-weight marker (labelled in kDa). (b) Purification of FapF. Superdex 200 (GE Healthcare) gel-filtration profile of FapF106–430 in 0.5% C8E4. The main peak at 65 ml corresponds to FapF106–430. Inset, SDS–PAGE of fractions from the FapF106–430 nickel purification: M, marker; F, flowthrough; W1, first wash; W2, second wash; E, elution peaks 1 and 2.