Abstract
Acetyl-Coenzyme A carboxylase (ACC), malonyl-CoA reductase (MCR), and malonic semialdehyde reductase (MRS) convert HCO3− and acetyl-CoA into 3-hydroxypropionate (3HP) in the 3-hydroxypropionate/4-hydroxybutyrate carbon fixation cycle resident in the extremely thermoacidophilic archaeon Metallosphaera sedula. These three enzymes, when introduced into the hyperthermophilic archaeon Pyrococcus furiosus, enable production of 3HP from maltose and CO2. Sub-optimal function of ACC was hypothesized to be limiting for production of 3HP, so accessory enzymes carbonic anhydrase (CA) and biotin protein ligase (BPL) from M. sedula were produced recombinantly in Escherichia coli to assess their function. P. furiosus lacks a native, functional CA, while the M. sedula CA (Msed_0390) has a specific activity comparable to other microbial versions of this enzyme. M. sedula BPL (Msed_2010) was shown to biotinylate the β-subunit (biotin carboxyl carrier protein) of the ACC in vitro. Since the native BPLs in E. coli and P. furiosus may not adequately biotinylate the M. sedula ACC, the carboxylase was produced in P. furiosus by co-expression with the M. sedula BPL. The baseline production strain, containing only the ACC, MCR, and MSR, grown in a CO2-sparged bioreactor reached titers of approximately 40 mg/L 3HP. Strains in which either the CA or BPL accessory enzyme from M. sedula was added to the pathway resulted in improved titers, 120 or 370 mg/L, respectively. The addition of both M. sedula CA and BPL, however, yielded intermediate titers of 3HP (240 mg/L), indicating that the effects of CA and BPL on the engineered 3HP pathway were not additive, possible reasons for which are discussed. While further efforts to improve 3HP production by regulating gene dosage, improving carbon flux and optimizing bioreactor operation are needed, these results illustrate the ancillary benefits of accessory enzymes for incorporating CO2 into 3HP production in metabolically engineered P. furiosus, and hint at the important role that CA and BPL likely play in the native production of 3HP in M. sedula.
Keywords: biotin protein ligase, carboxylase, carbon dioxide, Pyrococcus furiosus, 3-hydroxypropionate, Metallosphaera sedula
Introduction
The 3-hydroxypropionate/4-hydroxybutyrate (3HP/4HB) cycle is a carbon fixation pathway unique to the extremely thermoacidophilic archaeal order Sulfolobales, found in species such as Metallosphaera sedula (Berg et al. 2007; Berg et al. 2010). This cycle could be used as a basis for fuel and chemical production in a metabolic engineering host (Hawkins et al. 2013; Keller et al. 2013). The first part of this cycle, referred to as sub-pathway 1 (SP1), catalyzes the conversion of acetyl-CoA and bicarbonate to 3-hydroxypropionate (see Figure 1) and involves three enzymes: the heterotrimeric acetyl-CoA/propionyl-CoA carboxylase (ACC) (Msed_0147, 0148, and 1375) (Hügler et al. 2003); malonyl-CoA reductase (MCR) (Msed_0709) (Alber et al. 2006); and malonic semialdehyde reductase (MSR) (Msed_1993) (Kockelkorn and Fuchs 2009). The genes encoding the three enzymes of SP1 were inserted into the hyperthermophilic anaerobe Pyrococcus furiosus, a newly established metabolic engineering platform, to produce 3-hydroxypropionate (3HP) at titers of approximately 60 mg/L from maltose and CO2 (Keller et al. 2013).
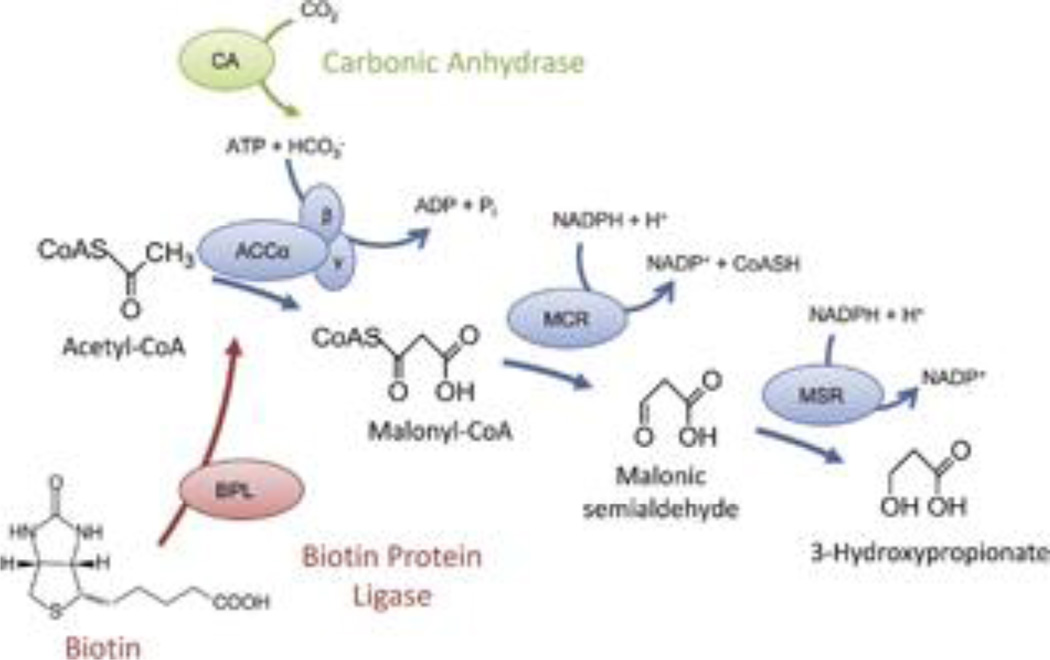
Figure 1.
3-Hydroxypropionate formation and accessory enzymes. The first three enzymes of the 3HP/4HB cycle (sub-pathway 1 [SP1]) catalyze the formation of 3HP from acetyl-CoA and bicarbonate. Two accessory enzymes are potentially involved in carboxylase function—CA catalyzes the hydration of dissolved CO2 gas to supply bicarbonate and BPL is required for covalently ligating biotin to the β-subunit of ACC. CA, carbonic anhydrase; BPL, biotin protein ligase; ACC, acetyl-CoA carboxylase; MCR, malonyl-CoA reductase; MSR, malonic semialdehyde reductase.
The genetic system developed for P. furiosus (Topt 100°C) is one of the most versatile available for any hyperthermophile, and has allowed for a variety of metabolic engineering efforts in this organism (Zeldes et al. 2015) P. furiosus cells grow quickly, and tolerate sudden shifts to much lower growth temperatures, allowing them to express pathways derived from less extreme thermophiles (Basen et al. 2012), such as the 3HP production pathway from M. sedula (Topt 75°C) discussed above. More generally, high temperature fermentations using a thermophilic host could save on cooling costs (which can be considerable for mesophilic fermentations), reduce the risk of contamination, and improve solubility of likely feedstocks, such as lignocellulose.
The successful demonstration of 3HP production in P. furiosus opens the door for further inquiry into the interactions between native host metabolism and the synthetic pathway to improve production titers. Understanding transcription and enzyme maturation, competing pathways, side reactions, redox and cofactor balance, and catalytic driving forces are all important for optimizing production and performance of any given synthetic pathway (Berríos-Rivera et al. 2002; Carothers et al. 2009; Shen et al. 2011). To this end, an improvement in overall 3HP production was achieved by the deletion of genes encoding an acetyl-CoA synthetase, a key enzyme for fermentative metabolism in P. furiosus and a competitor for the substrate molecule acetyl-CoA (Thorgersen et al. 2014). Also, through the insertion of two genes encoding accessory enzymes from M. sedula, a biotin protein ligase (BPL) and carbonic anhydrase (CA), P. furiosus strain MW76 produced 3HP titers of 276 mg/L in an agitated bioreactor in which CO2 sparging was used (Hawkins et al. 2015). However, the specific contributions of BPL and CA were not clear. This brings up the question as to what role CO2 incorporation (driven by CA) and carboxylase function (dependent on BPL) played in titer improvement.
Carboxylases, such as ACC, represent the key carbon-incorporating step in autotrophic growth and are typically the primary factor that determines energetic cost and environmental niche of a given carbon fixation cycle (Erb 2011). Therefore, both BPL and CA are expected to improve 3HP titers by increasing the efficiency of ACC in the SP1 production pathway (see Figure 1). ACC is a 560 kDa complex, comprised of biotin carboxylase (α-subunit: ACC-α), biotin carboxyl carrier protein (BCCP) (β-subunit: ACC-β), and carboxyl transferase (γ-subunit: ACC-γ), likely in a (αβγ)4 arrangement (Hügler et al. 2003). Biotin, or vitamin H, is an essential prosthetic group that is post-translationally attached at the active site of certain carboxyl-transferring enzymes (Streit and Entcheva 2003). BPL is responsible for attaching biotin to ACC-β (BCCP) to create active holoenzyme, so it is expected to increase carboxylase activity of the ACC by increasing the fraction of enzyme that is in its active holo-form. CA, on the other hand, catalyzes the interconversion of bicarbonate and dissolved CO2 in aqueous environments (CO2 + H2O ⇄ HCO3− + H+), thereby playing an essential role in CO2 transport out of metabolizing aerobic cells (Henry 1996). Its prevalence in autotrophic bacteria has been proposed to mimic CO2 concentrating mechanisms used by plants to facilitate carbon fixation (Smith et al. 1999). Bicarbonate, rather than CO2, is the substrate of ACC (Hügler et al. 2003), so by accelerating the normally slow equilibration between these two compounds, CA is expected to increase intracellular bicarbonate levels and thereby improve the kinetic rate of CO2 fixation by ACC.
P. furiosus cell extracts lack native carbonic anhydrase activity, so expression of M. sedula CA could improve bicarbonate availability. The native P. furiosus BPL may not biotinylate M. sedula ACC-β efficiently, due to sequence differences around the biotinylation site when compared to the native M. sedula version of this enzyme. To determine their relative contributions in 3HP-producing strains of P. furiosus, the CA and BPL were first characterized in vitro. Then, their in vivo impact on 3HP titers in P. furiosus was analyzed by comparing 3HP production strains containing one or both accessory enzymes. In addition to examining the ancillary contributions of each accessory enzyme to improvements in 3HP titer, prospects were considered for P. furiosus as a hyperthermophilic metabolic engineering platform for liquid fuels and chemicals production that includes CO2-incorporating steps.
Materials and Methods
P. furiosus strain construction
P. furiosus was routinely grown in serum bottles using either defined (3.5 g/L cellobiose, 1× amino-acid solution, 1× vitamin mix), or complex (5 g/L yeast extract, 5 g/L maltose) medium, both in a seawater-based medium containing 1× base salts, 1× trace minerals, 10 µM sodium tungstate, 0.25 mg/mL resazurin, 0.5 g/L cysteine, 0.5 g/L sodium sulfide, 1g/L sodium bicarbonate, and 1 mM potassium phosphate, all buffered to pH 6.8. For descriptions of stock solutions see (Lipscomb et al. 2011).
All P. furiosus transformations were done using uracil prototrophic selection on defined medium, essentially as previously described (Lipscomb et al. 2011), using linearized plasmids or linear splice overlap extension (SOE) PCR products. Strains were further purified by two consecutive transfers on solid medium, and the final strains were verified by PCR and sequencing of the regions containing the chromosomal insertions. The P. furiosus genome regions (3 and 5) used for chromosomal integration of the constructs contain little to no transcriptional activity, as determined from analysis of tiling array data (Yoon et al. 2011). Strains used and constructed in this study are listed in Table I.
Table I.
P. furiosus strains used in this study
| Strain | Parent | Genotype | Source |
|---|---|---|---|
| COM1 | DSM3638 | ΔpyrF | (Lipscomb et al. 2011) |
| MW112 | COM1 | ΔpyrF:PgdhpyrF-Ppep-BPL-CA-Pslp- ACCαβ-his6ACCγ |
This work |
| MW56 | COM1 | ΔpyrF:PgdhpyrF-Pslp-ACCαβγ- MCR-MSR |
(Keller et al. 2013) |
| MW76 | COM1 | ΔpyrF:PgdhpyrF-Ppep-BPL-CA-Pslp- ACCαβγ-MCR-MSR |
(Hawkins et al. 2015) |
| RMK120 | MW60 | ΔpyrF:PgdhpyrF-Ppep-BPL-Pslp- ACCαβγ-MCR-MSR |
This work (Thorgersen et al. 2014) |
| RMK121 | MW60 | ΔpyrF:PgdhpyrF-Ppep-CA-Pslp- ACCαβγ-MCR-MSR |
This work (Thorgersen et al. 2014) |
To construct strain RMK120, the Pgdh-pyrF-Ppep-BPL region, without the CA gene and Pslp-ACCα locus, were amplified by PCR from MW76 genomic DNA and combined using SOE PCR to generate the Pgdh-pyrF-Ppep-BPL-Pslp-ACCα construct. The SOE PCR construct was transformed into strain MW60 (Thorgersen et al. 2014), a markerless version of MW56 (Keller et al. 2013), to construct strain RMK120. To construct strain RMK121, the Pgdh-pyrF-Ppep and CA-Pslp-ACCα regions were amplified from MW76 genomic DNA, combined using SOE PCR to generate the Pgdh-pyrF-Ppep-CA-Pslp-ACCα construct, and transformed into strain MW60.
To clone and express 6×His-tagged ACC in P. furiosus, the artificial operons Ppep-BPL-CA and Pslp-ACCαβ-his6ACCγ were constructed and transformed into COM1 to generate strain MW112. PCR products of Ppep-BPL-CA, Pgdh-pyrF, Pslp-ACCαβ, his6ACCγ, and 0.5-kb upstream and downstream flanking regions to genome region 5 (between convergent genes PF1232 and PF1233) were combined via Gibson assembly (Gibson et al. 2009) (Gibson assembly kit, New England Biolabs, Ipswich, MA) to construct the plasmid pGL033. The Pslp-ACCαβ-his6ACCγ artificial operon contained a P. furiosus RBS from the gene encoding pyruvate ferredoxin oxidoreductase subunit γ (PF0791, 5'- ggaggtttgaag) upstream of the ACC γ-subunit gene; a 6×His tag flanked by two alanine codons (5'-gcacatcaccaccaccatcacgct) was also inserted after the start codon of the ACCγ gene to facilitate protein purification. Linearized pGL033 was transformed into COM1 to construct strain MW112.
Bioreactor growth of P. furiosus strains
Bioreactor media matched routine growth media, except for the addition of 0.25 mg/L biotin and omission of sodium bicarbonate. A pH probe was installed in the 3L Applikon glass bioreactors (ADI 1010/1025; Delft, The Netherlands) before autoclaving. Components for 1 L of media were added and the bioreactors were heated to 95°C, and gas sparging with 20% CO2, 80% N2 was initiated. Media pH was adjusted to 6.8 with NaOH, based on 10 mL samples taken from the hot bioreactors and quickly cooled to below 30°C. Probes for pH control were calibrated at room temperature, with pH offset determined using the cooled media samples. Gas flow rates were controlled using Matheson E910 rotameters (FM-1050 series; Basking Ridge, NJ, USA) and agitation came from Rushton impellers. Reactors were inoculated to 1×106 with cells grown for 10 to 12 hours in serum bottles. Growth was monitored by cell counts until density reached 1×108 cells/mL, then bioreactors were cooled by passing cold water through a heat exchange-port. During media heating and initial growth phase stir rate, temperature, and gas flow were 250 rpm, 95°C, and 40 mL/min, then changed to 400 rpm, 72°C, and 70 mL/min at the temperature switch. Samples consisted of 10 mL separated into a cell pellet, filter-sterilized supernatant, and 1 mL for cell counts. Samples were collected immediately following inoculation, immediately following temperature switch, 5 hours after temperature switch, and then twice daily up to 90 hours.
To assay for ACC activity in cell extracts (CEs), bioreactors of COM1, MW56, and RMK120 were grown as for the 3HP production experiments, but 24 hours after temperature switch the entire volume of the reactors was harvested. Cells were lysed in low osmotic buffer, and centrifuged at 24,000g for 30 minutes to remove insoluble components. ACC activity in the CE was determined by monitoring phosphate (described below).
Quantification of metabolites in bioreactors
Acetate, maltose, and 3HP in bioreactor supernatant samples were measured by HPLC using an Rezex-ROAcolumn, 300 mm × 7.8 mm ID (Phenomenex, Torrance, CA) heated to 60°C. The system consisted of a Waters (Milford, MA) 1525 Binary HPLC pump, with detection by Waters 2414 Refractive Index Detector (maltose) and Waters 2487 Dual λ Absorbance Detector (acetate and 3HP). Sulfuric acid was added to cell supernatants to 0.05% v/v, and 40 µL injections were run in 5 mM sulfuric acid mobile phase at 0.6 mL/min for 50 minutes. Standards were made in distilled water and sulfuric acid added to 0.05%.
Heterologous expression of M. sedula genes in E. coli
The gene encoding the putative biotin protein ligase in the M. sedula genome (Msed_2010) was amplified from genomic DNA and cloned into pET-46 Ek/LIC vector with an N-terminal 6×His-tag. The gene encoding the putative carbonic anhydrase in the M. sedula genome (Msed_0390) was amplified from genomic DNA and cloned using primers designed to introduce an XhoI restriction site after the stop codon. Then the PCR product was purified and cloned into pET21b (+) vector with a C-terminal 6×His-tag. The M. sedula genes encoding the α-, β-, and γ-subunits of the carboxylase (Msed_0147, Msed_1048, and Msed_1375) were cloned by a ligation-independent method using pET46 Ek/LIC, pRSF-2 Ek/LIC, and pCDF-2 Ek/LIC (EMD4 Biosciences), respectively, which are compatible vectors enabling co-expression of these genes in various combinations: αβγ (N-terminal 6×His on γ), αβ (N-terminal 6×His on β), βγ (N-terminal 6×His on γ), or β only (N-terminal 6×His) in a single E. coli strain. All vectors containing target genes were transformed into competent E. coli Rosetta 2 (DE3) cells (EMD Millipore, Darmstadt, Germany). Cells were grown in 1L LB media, containing 100 µg ml−1 ampicillin (for pET46 and pET21b), 30 µg ml−1 kanamycin (for pRSF-2), 50 µg ml−1 streptomycin (for pCDF-2), and 34 µg ml−1 chloramphenicol (to maintain the Rosetta plasmid), according to the different antibiotic resistance markers carried by the plasmids. Cells were harvested by centrifugation 4–5 hours after induction with 0.1–0.2 mM IPTG, and stored at −20°C.
Purification of recombinant His-tagged M. sedula proteins
E. coli cells containing recombinant M. sedula proteins were re-suspended in 20 mM sodium phosphate, 0.5 M NaCl, pH 7.4, and disrupted by sonication for 10 min at 60% Amplitude (10 sec on, 10 sec off). Cell extract was incubated at 65°C for 20 min to denature E. coli native proteins, and centrifuged at 16,000 × g for 15 min. The heat-treated cell extract was loaded onto a 5 mL HiTrap IMAC HP column (GE Healthcare Life Sciences), equilibrated with 20 mM sodium phosphate, 0.5 M NaCl, at pH 7.4. The column was washed with five bed volumes of equilibration buffer and eluted with 300 mM imidazole at a flow rate of 1 ml min−1. Active fractions eluted with imidazole were pooled and dialyzed in 50 mM Tris-HCl, pH 8.2 to remove imidazole. The pooled, dialyzed fractions were concentrated via Amicon® Ultra-15 Centrifugal Filter Units – 3 kDa (EMD Millipore) and stored at −80°C.
Expression and purification of recombinant M. sedula ACC from P. furiosus
Strain MW112 was routinely grown in bioreactors in growth medium containing 5 g/L maltose, 5 g/L yeast extract, 5 g/L tryptone, and 0.25 mg/L biotin. Two liters of culture were grown at 95°C with 15 mL/min N2 (80%)/CO2 (20%) sparging and 250 rpm agitation to a density of 108 cells/mL. Then, temperature was reduced to 72°C and cells were grown for 18 hours before being harvested by centrifugation.
Cells were lysed by re-suspending in low salt buffer (50 mM Tris-HCl, pH 8.2) and stirring at room temperature for 2 hours. The cell lysate was centrifuged at 10,000 × g for 20 min, and the supernatant was collected and filter-sterilized, before being loaded on a 1 mL HiTrap IMAC HP column (GE Healthcare Life Sciences) equilibrated with 50 mM Tris, 100 mM NaCl, pH 8.0. The column was washed with five bed volumes of equilibration buffer and eluted with 500 mM imidazole at a flow rate of 1 ml min−1. Fractions were pooled, dialyzed, and concentrated as described above.
The native molecular mass of the enzyme was estimated by gel filtration chromatography. Protein from the IMAC step was applied to a Superdex 75 HR 16/60 gel filtration column (GE Healthcare Life Sciences; volume, 20 ml), which had been equilibrated with 50 mM sodium phosphate, 100 mM NaCl, pH 7.2. The flow rate was 0.5 ml min−1. The column was calibrated with the following molecular mass standards (Sigma): β-Amylase from sweet potato (200 kDa), alcohol dehydrogenase from yeast (150 kDa), bovine serum albumin (66 kDa), carbonic anhydrase from bovine erythrocytes (29 kDa), cytochrome c from horse heart (12.4 kDa), and aprotinin from bovine lung (6.5 kDa). Proteins were visualized using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) 4–12% gels (Invitrogen) and GelCode Blue stain (ThermoFisher).
Biochemical assays
In vitro biotinylation
Biotin protein ligase (BPL) activity was measured by following biotinylation of purified recombinant E1-β or E1-βγ. Assay conditions were adapted according to literature reports (Chapman-Smith et al. 1999). The assay solution contained 30 mM Tris, pH 8.2, 100 mM KCl, 5 mM MgCl2, 0.5 mM DTT, 0.3 mM ATP, 10 µM biotin and 1 µM purified recombinant putative BPL (Msed_2010). The reaction was initiated by the addition of purified recombinant E1-β or E1-βγ to a final concentration of 2.5 µM and incubated for 30 min at 70°C. As a control, E1-β or E1-βγ were incubated with BPL storage buffer instead. After incubation, samples were filtered through a 10-kDa molecular weight cutoff filter (YM-10 Thermo Scientific) to remove excess biotin and re-dissolved in 50 mM Tris-HCl, pH 8.2. A biotin capture method, based on streptavidin sepharose beads (GE Healthcare), was developed to visualize biotinylated proteins on SDS-PAGE. The products from in vitro biotinylation (including control) were loaded onto streptavidin sepharose beads and unbiotinylated protein was washed away. Bound protein was released by heating the beads at 95°C for 5 min to denature the streptavidin. Both bound and unbound fractions were then run on SDS-PAGE for visualization.
Acetyl-CoA carboxylase
ACC activity was assayed using a coupled assay with MCR. The assay mixture, containing 80 mM MOPS/NaOH pH 8.0, 0.5 mM acetyl-CoA, 5 mM MgCl2, 5 mM 1,4-dithioerythritol, 5 mM ATP, 10 mM NaHCO3, 0.5 mM NADPH, and purified recombinant MCR, was heated to 70°C. Then, purified recombinant ACC was added, and NADPH consumption (by MCR acting on the product of ACC, malonyl-CoA) was monitored continuously by absorbance at 340 nm.
A discontinuous assay was also used that relied on a phosphate colorimetric assay kit (BioVision, Milpitas, CA) to detect free phosphate release by ATP hydrolysis catalyzed by ACC. The reaction solution contained 75 mM MOPS/NaOH pH 8.0, 0.5 mM acetyl-CoA, 5 mM MgCl2, 5 mM 1,4-dithioerythritol, 2 mM ATP, and 10 mM NaHCO3, and was incubated at 70°C prior to addition of ACC. Samples were taken regularly for up to 6 minutes and assayed for phosphate.
Carbonic anhydrase
The CO2 hydration assay followed a modified version of the Wilbur-Anderson method (Wilbur and Anderson 1948). CO2-saturated water was made by subliming dry ice in a glass bottle for 30 min, with the bottle sealed with a rubber stopper to prevent leakage. The CO2-saturated water (3 ml) was immediately added to 2 ml of Tris–Sulfate buffer (100 mM; pH 8.3) containing 0.5 ml of enzyme solution. The enzyme solution was diluted and the reaction carried out at low temperature (10°C) to promote CO2 solubility and keep reaction rates within measurable limits. The time (t) required for the pH to drop from 8.0 to 6.3, as catalyzed by the enzyme, was measured. The control for the pH change (8.0–6.3) used enzyme storage buffer (50 mM Tris-HCl, pH 8.2) substituted for the enzyme solution. The Wilbur–Anderson Units were determined from the equation: 1U = (tc−t)/t, where tc is the time for control. Protein content was determined by Bradford assay and the activity was reported in U/mg protein. All measurements were carried out in triplicate.
Results
Identification and characterization of the M. sedula carbonic anhydrase (CA)
The M. sedula genome encodes two putative CAs (Msed_0390 and Msed_1618), neither of which has previously been characterized biochemically. Msed_0390 was significantly up-regulated under autotrophic growth compared to heterotrophic growth (Auernik and Kelly 2010), responding in concert with transcription of genes encoding 3HP/4HB carbon fixation cycle enzymes. Msed_1618 did not respond under any growth conditions tested and was transcribed at very low levels; as such, it was not considered further for the purposes of this study. Msed_0390 was annotated as a β-class carbonic anhydrase, most likely required for efficient uptake of inorganic carbon by M. sedula for carbon fixation. This CA was suspected to play a vital role as a complementary enzyme for the M. sedula 3HP/4HB cycle to increase activity of CO2 fixation by rapidly providing bicarbonate to acetyl-CoA carboxylase.
The heat-treated cell extract of recombinant E. coli expressing Msed_0390 exhibited CO2 hydration activity, relative to the control. Furthermore, the IMAC purified recombinant enzyme had a CO2 hydration activity of 292 U/mg at 10°C, validating that Msed_0390 encodes a functional CA for CO2 and HCO3− interconversion in M. sedula. This enzyme is the first CA that has been characterized from extremely thermoacidophilic archaea. The predicted molecular mass of the CA is 22 kDa (including a C-terminal 6×His tag), which is consistent with the Mr of 20.6 kDa determined by size exclusion chromatography, suggesting that this enzyme functions as a monomer (Table II). Thus, the molecular assembly of this M. sedula CA differs from previously characterized β-CAs that typically exist as homodimers, homotetramers and homooctamers, with the fundamental structural unit as a dimer (Rowlett 2010). M. sedula grows at low pH (pH 2) where CO2 has low solubility, but the cytoplasm, where the 3HP/4HB cycle utilizes bicarbonate as a substrate, is neutral (Peeples and Kelly 1995). There is no evidence that the M. sedula β-CA is an extracellular or membrane-bound protein (no discernible signal peptide). So, it most likely functions in the cytoplasm to accelerate the hydration of CO2 to carbonic acid, which is then rapidly deprotonated to bicarbonate at neutral pH (pKa1 = 6.4) (Loerting and Bernard 2010).
Table II.
Recombinant M. sedula enzymes used in this study
| Enzyme | Native Molecular Mass (kDa) |
Subunit Molecular Mass (kDa) |
Assembly | Comments |
|---|---|---|---|---|
| Acetyl-CoA carboxylase |
507 | α - 57 | (αβγ)4 | Agrees with native form (Hügler et al. 2003) |
| β - 18.6 | ||||
| γ - 57 | ||||
| Carbonic anhydrase |
22 | 22 | α1 | Most β-CAs are heteromultimers, but Msed_0390 is monomeric |
| Biotin protein ligase |
57 | 26 | α2 |
S. tokodaii homolog is monomeric (Li et al. 2006) |
P. furiosus does not appear to natively express a functional CA to aid in the conversion of CO2 to bicarbonate, the substrate of the 3HP/4HB cycle. So far, the only evidence for CA in P. furiosus was the detection of cross-reacting proteins in P. furiosus cell extract to antisera raised from previously characterized mesophilic CAs in Western blots (Smith et al. 1999). Here, the P. furiosus cell extract exhibited no detectable CA activity (< 0.01 Wilbur-Anderson unit at 55°C). It should be noted that the upper temperature limit of the assay (55°C) is well below the expected activity optimum for a putative P. furiosus CA, and the decreased solubility of CO2 at higher temperatures under atmospheric pressure impedes determination of CA activity. A crystal structure of a γ-class CA in P. horikoshii has been reported, but no activity was noted for this enzyme (Jeyakanthan et al. 2008). Therefore, the presence of a putative wild-type P. furiosus CA for efficient CO2 uptake and bicarbonate conversion is unlikely.
Assuming that P. furiosus lacks CA activity, the production of the bicarbonate substrate for the M. sedula ACC in the cytoplasm of the engineered strain is limited to the rate of uncatalyzed conversion from CO2, creating a potential bottleneck in the recombinant 3HP production pathway. To overcome this bottleneck, the gene encoding CA from M. sedula was inserted into P. furiosus, generating strain RMK121. This could then be compared to P. furiosus strain MW56, which lacks the M. sedula CA, to determine the role that bicarbonate availability plays in ACC function.
Production and characterization of recombinant carboxylase ACC
Previously, a partially purified version of the native M. sedula ACC was characterized to confirm its function (Hügler et al. 2003). Since a recombinant version of this enzyme was recruited to P. furiosus strains producing 3HP, efforts were made to express the recombinant ACC in active form to evaluate its biochemical properties. In order to evaluate the in vitro activity of the ACC, the individual subunits were expressed in separate E. coli strains as well as co-expressed on separate plasmids, but in the same strain. In both cases, the recombinant ACC α and γ subunits were recovered only in the insoluble fraction, and furthermore lacked catalytic activity. Hulger et al. expressed only the ACC-γ subunit in E. coli, and saw no change in activity when it was added to the purified native protein [Hugler et al., 2003 DOI: 10.1046/j.1432-1033.2003.03434.x). The fact that they do not report a fully recombinant ACC may indicate that the authors encountered similar difficulties in expressing the functional holoenzyme in E. coli.
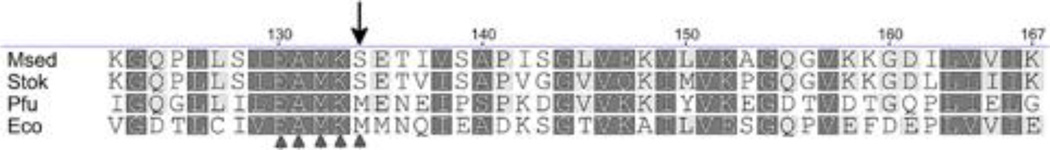
A biotin quantitation assay indicated that the ACC-β subunit was not being properly biotinylated in E. coli. The biotin cofactor is attached post-translationally to a specific lysine residue on ACC-β by BPL (Clarke et al. 2003). Analysis of the amino acid sequence in the vicinity of the lysine residue targeted by BPL provided some insights into the problem. While similar biotinylated proteins in both E. coli and P. furiosus have a methionine immediately C-terminal to the biotinylated lysine residue, S. tokodaii, M. sedula and the other Sulfolobales have a serine (Li et al. 2006) (Figure 2). In S. tokodaii, mutation of the serine to methionine allowed biotinylation by E. coli’s BPL, and S. tokodaii BPL could target the E. coli enzyme if its methionine was mutated to serine, although still less efficiently than their native substrates (Li et al. 2006; Sueda et al. 2006). Therefore, it seemed likely that the amino acid sequence of M. sedula’s ACC-β would make it difficult for the BPL of either E. coli or P. furiosus to biotinylate it efficiently.
Figure 2.
Amino acid sequence alignment of BCCP around the canonical lysine residue (K). The conserved—E-X-M-K-M-motif is indicated by triangles. Mutated site in the Sulfolobales is marked by arrow. Msed, M. sedula Msed0148, Stok, S. tokodaii ST0592, Pfu, P. furiosus PF0673, Eco, E. coli ECs4127.
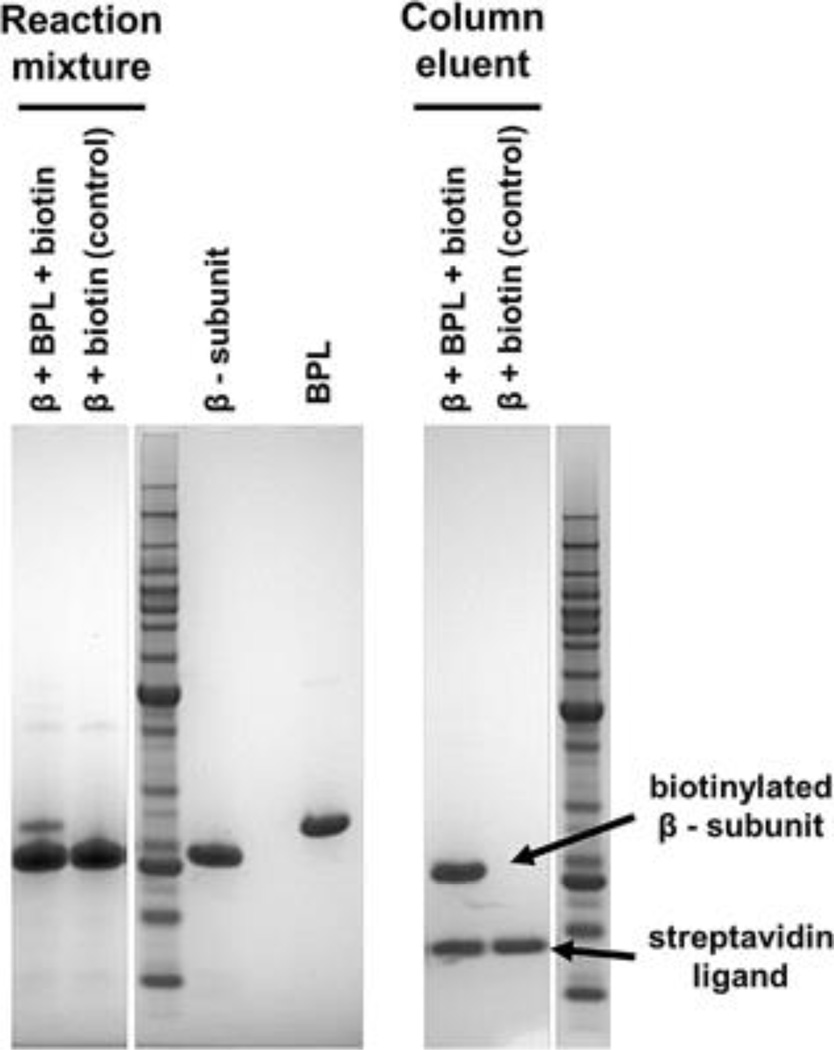
Rather than mutate the M. sedula ACC-β subunit so it could be modified by the native BPL in P. furiosus, the gene encoding the M. sedula BPL was expressed in P. furiosus. The sequence for BPL from S. tokodaii (Li et al., 2006) was used to query the NCBI database, and Msed_2010, with 56% identity at the amino acid level, appeared to be a homolog of the S. tokodaii BPL. To confirm, Msed_2010 was expressed in E. coli and incubated with ACC-β expressed in E. coli in the presence of biotin, where it was able to biotinylate ACC-β (Figure 3). The predicted molecular mass of the M. sedula BPL is 26 kDa, yet the molecular mass determined by size exclusion chromatography was 57 kDa. Therefore, the M. sedula BPL probably functions as a homodimer (Table II).
Figure 3.
In vitro biotinylation of recombinant Msed ACC-β with recombinant Msed BPL. Purified M. sedula ACC-β and BPL were combined with biotin cofactor and incubated for 30 min at 70°C. This “reaction mixture” was applied to a streptavidin column and washed. Bound fraction consisting of biotinylated ACC-β was released from the beads by heat denaturation.
Since Msed_2010 was able to biotinylate ACC in vitro, it was cloned into P. furiosus along with his-tagged ACC (N-terminus of γ-subunit) to generate strain MW112. Recombinant ACC purified from MW112 cell extract (Table II) was functional, although less active than the native enzyme with a specific activity of 0.65 µmol/min/mg compared to 3.2 µmol/min/mg for the native-purified enzyme (Hügler et al. 2003). SDS-PAGE indicated low expression of ACC-β, which may account for some of the loss in activity. The functional production of M. sedula ACC in P. furiosus agrees with previous results; heterologous expression of protein in a more closely related host (in this case another archaeal extreme thermophile) can yield active enzyme when production in E. coli fails (Mueller et al. 2009).
Since P. furiosus strain MW56, which lacks the M. sedula BPL (Msed_2010), was able to produce 3HP, the ACC must be biotinylated to some extent by the native P. furiosus BPL. P. furiosus strain RMK120 was constructed with M. sedula BPL in addition to SP1 for 3HP production, to determine whether production would improve with more effective biotinylation of the ACC. As shown in Table IV (or expand Table III ?), ACC activity in cell-extract of RMK120 is approximately double that of MW56, despite being under control of the same consitutive promoter. This confirms that the more efficient biotinylation accomplished by Msed BPL results in increased ACC activity. Note that the control strain COM1 showed about half as much activity as MW56, which, since it lacks ACC altogether, must be the result of background ATP consumption in the CE. Therefore, these specific activities are likely overestimates.
Table IV.
Activity of ACC measured in cell extract of bioreactor grown cells
| Strain | Genes | Average N=3 (nmol/min/mg) ± SD |
|---|---|---|
| COM1 | - | 89 ± 20 |
| MW56 | SP1 | 161 ± 55 |
| RMK120 | SP1 + BPL | 344 ± 29 |
Table III.
Maximum 3HP titers reached during bioreactor runs with recombinant strains
| Strain | Genes | Average N=3 (mg/L) ± SD | Titer relative to MW56 |
|---|---|---|---|
| MW56 | SP1 | 44 ± 11 | 1.0 |
| MW76 | SP1 + BPL + CA | 236 ± 18 | 5.3 |
| RMK120 | SP1 + BPL | 366 ± 74 | 8.2 |
| RMK121 | SP1 + CA | 119 ± 21 | 2.7 |
Effect of recombinant M. sedula CA and BPL on 3HP production in P. furiosus
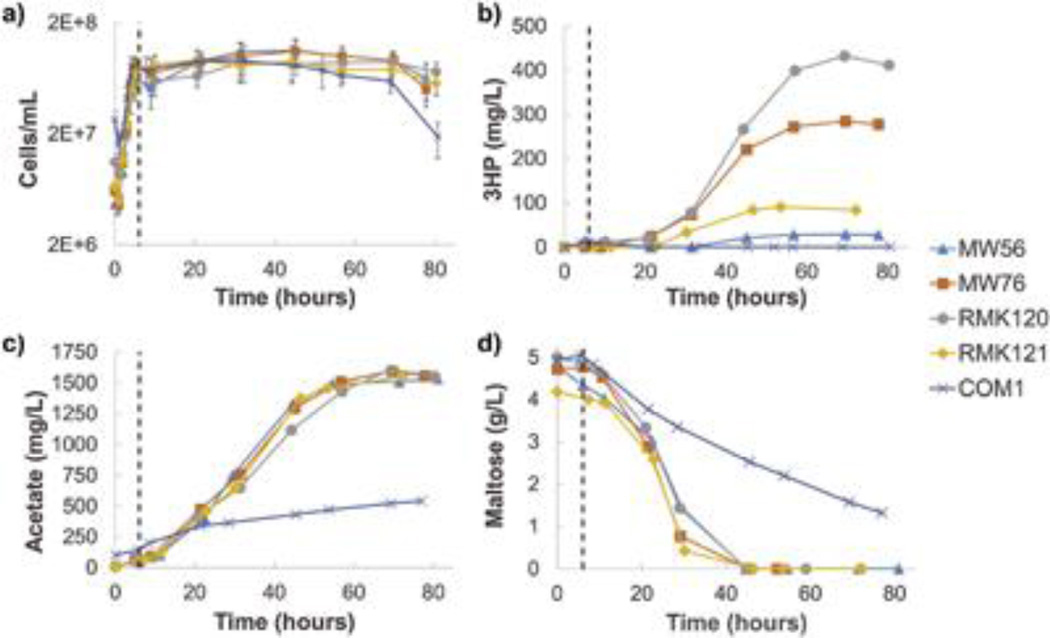
To determine the separate and potential complementary roles of CA and BPL in production of 3HP, four metabolically engineered P. furiosus strains and the unmodified parent strain COM1 were grown in 1L bioreactors. All engineered strains, including MW56, contained the three M. sedula enzymes making up SP1 (ACC, MCR and MRS), while RMK120 also contained BPL, RMK121 also contained CA, and MW76 contained both BPL and CA. Cells were grown at 95°C until they reached a density of 1×108 cells/mL, at which time the temperature was reduced to 72°C to match the optimum temperature of the recombinant M. sedula enzymes. Following the temperature shift, cells went from doubling every hour at 95°C to every 10–20 hours at 72°C (only about one more doubling occurs after temperature shift) (Figure 4a). Despite relatively constant cell number, cell size increased dramatically following the switch to 72°C, as has been noted previously (Basen et al. 2012). Cell pellets taken during bioreactor operation show a concomitant increase in size. Production of 3HP becomes evident for the engineered strains within 10 to 20 hours after temperature shift, and levels off once the maltose is consumed (Figure 4b). This lag in 3HP production agrees with previous experiments (Hawkins et al. 2015; Keller et al. 2013), and likely is related to the time it takes for the recombinant polypeptides to be synthesized and folded. Maximum 3HP titers were 44 mg/L in MW56 (SP1) and 236 mg/L in MW76 (SP1 + BPL +CA), consistent with previous results, i.e., 60 mg/L found by Keller et al. and 276 mg/L found by Hawkins el al., respectively. As expected, the control strain COM1 produced no detectable 3HP.
Figure 4.
Representative bioreactor runs of metabolically engineered P. furiosus strains. a) Cell counts, showing difference in growth rate before and after temperature shift from 95°C to 72°C. Time course of concentrations of b) 3HP, c) acetate, and d) maltose in supernatant. Vertical black line indicates temperature switch from 95°C to 72°C.
Figure 4c shows acetate production over the course of cell growth in bioreactors. In the absence of elemental sulfur, P. furiosus obtains all cellular energy from fermenting sugars to acetate, H2, and CO2 (Adams et al. 2001), so acetate levels provide a direct representation of the level of metabolic activity occurring in each strain. The only significant difference in acetate production is in the parent strain (COM1) control, which produced less than half as much acetate as all the engineered strains. COM1 was also the only strain that had residual unconsumed maltose left over at the end of the run (Figure 4d). So, it appears that, while the parent strain goes dormant and reduces metabolic activity at 72°C, the modified strains are forced to maintain active metabolism due to the use of the strong constitutive S-layer protein promoter (Pslp), and the resulting expression of the energy-intensive SP1 enzymes that remain active at the lower temperature. This result agrees with previous transcriptional data indicating genes related to energy metabolism and protein synthesis were up-regulated in MW76 relative to COM1 at 72°C (Hawkins et al. 2015).
Figure 4 shows representative experimental results for each strain, while Table III gives average max 3HP titers of triplicate bioreactor experiments with each strain. The parent strain (COM1) produced no 3HP, while of the engineered strains, RMK120 (SP1 + BPL) produced the most, followed by MW76 (SP1 + CA + BPL), then RMK121 (SP1 + CA), and finally MW56 (SP1), which served as a baseline for the productivity of the SP1 pathway without accessory enzymes. The finding that RMK120 (SP1+ BPL) was the most productive was unexpected, since it had only one of the two accessory enzymes hypothesized to improve productivity.
Discussion
Two accessory enzymes, important for optimal carboxylase function in the native 3HP/4HB carbon fixation cycle of M. sedula, were each examined in their recombinant forms. The role of these accessory enzymes in their native context is important to understand when inserting heterologous pathways into new hosts, as essential biochemical modifications may not occur in the host organism. Here, we report the successful production of recombinant M. sedula ACC using P. furiosus as the host, since no active ACC could be produced in E. coli. The key factor was the co-expression of the M. sedula BPL, which was highly specific for biotinylation of the M. sedula BCCP (ACC-β); neither the native E. coli nor the native P. furiosus BPL appeared to function as efficiently. This helps to explain why the P. furiosus strain lacking M. sedula BPL (MW56) produced less 3HP than the M. sedula BPL-containing strains (MW76 and RMK120) (Table III).
About a 3-fold improvement in 3HP titers could be achieved with the addition of the M. sedula CA to the P. furiosus strain containing the five genes encoding SP1 (MW56). The recombinant M. sedula CA produced in E. coli had an activity of 292 U/mg measured at 10°C. This value is comparable to the 720 U/mg (estimated kcat of 6.1 × 104 s−1) at 25°C reported for recombinant CA from another thermophilic archaeon, Methanosarcina thermophila (Alber et al. 1999), especially if consideration is given to assay temperature and enzyme temperature optimum. Carbonic anhydrases are reported to be among the most catalytic enzymes known, often limited by the diffusion rate of their substrate; even less active versions have kcat values on the order of 104 s−1 (Smith and Ferry 2000). Based on the similarity to the Me. thermophila CA, the M. sedula CA would also have a kcat on order of 104 s−1. Since the native M. sedula ACC has a reported kcat of 28 s−1, it is unlikely that the CA-catalyzed production of bicarbonate would be rate-limiting for carbon uptake in M. sedula. However, in P. furiosus, which lacks a functional CA, the comparatively slow uncatalyzed conversion of CO2 to HCO3− does appear to impair 3HP production.
It was interesting that the strain with both CA and BPL (MW76), while yielding 5-fold higher titers than the strain lacking both enzymes (MW56), produced significantly less 3HP as the strain with only BPL (RMK120) that produced 8-fold higher levels of 3HP than MW56. Clearly, under the conditions tested here, the effects of BPL and CA expression were not additive, and may in fact be antagonistic. There are several possible explanations for this unexpected effect. First, because strong constitutive promoters were used for the expression of CA and BPL in the recombinant P. furiosus strains, it is possible that the added burden of CA expression reduced BPL production in MW76 relative to RMK120. Improvements in productivity have been considered for other metabolically engineered pathways in extreme thermophiles, where reaction kinetics models suggested adjusting gene dosage can lead to improved product titers, as well as selectivity of desired to undesired products (Loder et al. 2015). A second possible explanation is that protein-protein interactions between the CA and ACC interfere with biotinylation of ACC by the BPL. In autotrophic bacteria, association of the CA with the carboxysome facilitates substrate channeling to ribulose-1,5-bisphosphate carboxylase/oxygenase, allowing increased carbon fixation at low CO2 concentrations (Bobik 2006). A similar mechanism may operate in M. sedula, considering the poor solubility of bicarbonate at its low pH growth optimum. If CA associates with ACC, high-level expression of CA in strain MW76 could cause tighter association, which may interfere with biotinylation of ACC by the BPL, leading to lower ACC activity than when BPL is expressed without CA, as in strain RMK120. Further work directed at improving titers and yields for 3HP in P. furiosus will examine this possibility and, perhaps, mitigate the deleterious effect by adjusting gene dosage through judicious choice of promoters.
The results demonstrate the importance of ancillary proteins on the function of heterologously expressed pathway components in metabolic engineering efforts. Failure to understand and account for these kinds of deficiencies can significantly compromise product titers and volumetric productivities. This effort also supports the promise of P. furiosus as a metabolic engineering platform for formation of bio-based products that incorporate CO2. The establishment of extreme thermophiles as widely used industrial microorganisms will depend on further advances that allow for their unique physiological properties to be strategically utilized.
Acknowledgments
This work was supported with grants to RMK and MWWA by the US Department of Energy Research ARPA-E Electrofuels Program (DE-AR0000081) and the US National Science Foundation (CBET-1264052, CBET-1264053). ABH acknowledges support from a US Department of Education GAANN Fellowship. AJL and BMZ acknowledge support from NIH Biotechnology Traineeships (2T32GM008776).
REFERENCES CITED
- Adams MW, Holden JF, Menon AL, Schut GJ, Grunden AM, Hou C, Hutchins AM, Jenney FE, Jr, Kim C, Ma K, et al. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 2001;183(2):716–724. doi: 10.1128/JB.183.2.716-724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alber B, Olinger M, Rieder A, Kockelkorn D, Jobst B, Hügler M, Fuchs G. Malonyl-coenzyme A reductase in the modified 3-hydroxypropionate cycle for autotrophic carbon fixation in archaeal Metallosphaera and Sulfolobus spp. J Bacteriol. 2006;188(24):8551–8559. doi: 10.1128/JB.00987-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alber BE, Colangelo CM, Dong J, Stålhandske CM, Baird TT, Tu C, Fierke CA, Silverman DN, Scott RA, Ferry JG. Kinetic and spectroscopic characterization of the gamma-carbonic anhydrase from the methanoarchaeon Methanosarcina thermophila. Biochemistry. 1999;38(40):13119–13128. doi: 10.1021/bi9828876. [DOI] [PubMed] [Google Scholar]
- Auernik KS, Kelly RM. Physiological versatility of the extremely thermoacidophilic archaeon Metallosphaera sedula supported by transcriptomic analysis of heterotrophic, autotrophic, and mixotrophic growth. Appl Environ Microb. 2010;76(3):931–935. doi: 10.1128/AEM.01336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basen M, Sun J, Adams MW. Engineering a hyperthermophilic archaeon for temperature-dependent product formation. mBio. 2012;3(2):e00053–e00512. doi: 10.1128/mBio.00053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg IA, Kockelkorn D, Buckel W, Fuchs G. A 3-Hydroxypropionate/4-Hydroxybutyrate autotrophic carbon dioxide Assimilation pathway in archaea. Science. 2007;318(5857):1782–1786. doi: 10.1126/science.1149976. [DOI] [PubMed] [Google Scholar]
- Berg IA, Kockelkorn D, Ramos-Vera WH, Say RF, Zarzycki J, Hugler M, Alber BE, Fuchs G. Autotrophic carbon fixation in archaea. Nat Rev Microbiol. 2010;8(6):447–460. doi: 10.1038/nrmicro2365. [DOI] [PubMed] [Google Scholar]
- Berríos-Rivera SJ, Bennett GN, San K-Y. Metabolic engineering of Escherichia coli: increase of NADH availability by overexpressing an NAD(+)-dependent formate dehydrogenase. Metab Eng. 2002;4(3):217–229. doi: 10.1006/mben.2002.0227. [DOI] [PubMed] [Google Scholar]
- Bobik TA. Polyhedral organelles compartmenting bacterial metabolic processes. Appl Microbiol Biotechnol. 2006;70(5):517–525. doi: 10.1007/s00253-005-0295-0. [DOI] [PubMed] [Google Scholar]
- Carothers JM, Goler JA, Keasling JD. Chemical synthesis using synthetic biology. Curr Opin Biotechnol. 2009;20(4):498–503. doi: 10.1016/j.copbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Chapman-Smith A, Morris TW, Wallace JC, Cronan JE. Molecular recognition in a post-translational modification of exceptional specificity. Mutants of the biotinylated domain of acetyl-CoA carboxylase defective in recognition by biotin protein ligase. J Biol Chem. 1999;274(3):1449–1457. doi: 10.1074/jbc.274.3.1449. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, Coulson J, Baillie R, Campopiano DJ. Biotinylation in the hyperthermophile Aquifex aeolicus. Eur J Biochem. 2003;270(6):1277–1287. doi: 10.1046/j.1432-1033.2003.03493.x. [DOI] [PubMed] [Google Scholar]
- Erb TJ. Carboxylases in natural and synthetic microbial pathways. Appl Environ Microbiol. 2011;77(24):8466–8477. doi: 10.1128/AEM.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Gouet P, Robert X, Courcelle E. ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 2003;31(13):3320–3323. doi: 10.1093/nar/gkg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins AB, Lian H, Zeldes BM, Loder AJ, Lipscomb GL, Schut GJ, Keller MW, Adams MWW, Kelly RM. Bioprocessing analysis of Pyrococcus furiosus strains engineered for CO2-based 3-hydroxypropionate production. Biotechnol Bioeng. 2015;112(8):1533–1543. doi: 10.1002/bit.25584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins AS, McTernan PM, Lian H, Kelly RM, Adams MWW. Biological conversion of carbon dioxide and hydrogen into liquid fuels and industrial chemicals. Curr Opin Biotech. 2013;24(3):376–384. doi: 10.1016/j.copbio.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Henry RP. Multiple roles of carbonic anhydrase in cellular transport and metabolism. Annu Rev Physiol. 1996;58:523–538. doi: 10.1146/annurev.ph.58.030196.002515. [DOI] [PubMed] [Google Scholar]
- Hügler M, Krieger RS, Jahn M, Fuchs G. Characterization of acetyl-CoA/propionyl-CoA carboxylase in Metallosphaera sedula Carboxylating enzyme in the 3-hydroxypropionate cycle for autotrophic carbon fixation. Eur J Biochem. 2003;270(4):736–744. doi: 10.1046/j.1432-1033.2003.03434.x. [DOI] [PubMed] [Google Scholar]
- Jeyakanthan J, Rangarajan S, Mridula P, Kanaujia SP, Shiro Y, Kuramitsu S, Yokoyama S, Sekar K. Observation of a calcium-binding site in the gamma-class carbonic anhydrase from Pyrococcus horikoshii. Acta Cryst D. 2008;64(Pt 10):1012–1019. doi: 10.1107/S0907444908024323. [DOI] [PubMed] [Google Scholar]
- Keller MW, Schut GJ, Lipscomb GL, Menon AL, Iwuchukwu IJ, Leuko TT, Thorgersen MP, Nixon WJ, Hawkins AS, Kelly RM, et al. Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide. Proc Natl Acad Sci U S A. 2013;110(15):5840–5845. doi: 10.1073/pnas.1222607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockelkorn D, Fuchs G. Malonic Semialdehyde Reductase, Succinic Semialdehyde Reductase, and Succinyl-Coenzyme A Reductase from Metallosphaera sedula: Enzymes of the Autotrophic 3-Hydroxypropionate/4-Hydroxybutyrate Cycle in Sulfolobales. J Bacteriol. 2009;191(20):6352–6362. doi: 10.1128/JB.00794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-Q, Sueda S, Kondo H, Kawarabayasi Y. A unique biotin carboxyl carrier protein in archaeon Sulfolobus tokodaii. FEBS Lett. 2006;580(6):1536–1540. doi: 10.1016/j.febslet.2006.01.083. [DOI] [PubMed] [Google Scholar]
- Lipscomb GL, Stirrett K, Schut GJ, Yang F, Jenney FE, Scott RA, Adams MWW, Westpheling J. Natural competence in the hyperthermophilic archaeon Pyrococcus furiosus facilitates genetic manipulation: construction of markerless deletions of genes encoding the two cytoplasmic hydrogenases. Appl Environ Microb. 2011;77(7):2232–2238. doi: 10.1128/AEM.02624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder AJ, Zeldes BM, Garrison GD, Lipscomb GL, Adams MWW, Kelly RM. Alcohol selectivity in a synthetic thermophilicn-butanol pathway is driven by biocatalytic and thermostability characteristics of constituent enzymes. Appl Environ Microbiol. 2015;81(20):7187–7200. doi: 10.1128/AEM.02028-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerting T, Bernard J. Aqueous carbonic acid (H2CO3) Chem Phys Chem. 2010;11(11):2305–2309. doi: 10.1002/cphc.201000220. [DOI] [PubMed] [Google Scholar]
- Mueller M, Takemasa R, Schwarz A, Atomi H, Nidetzky B. “Short-chain” α-1,4-glucan phosphorylase having a truncated N-terminal domain: Functional expression and characterization of the enzyme from Sulfolobus solfataricus. BBA-Proteins Proteom. 2009;1794(11):1709–1714. doi: 10.1016/j.bbapap.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Peeples TL, Kelly RM. Bioenergetic Response of the Extreme Thermoacidophile Metallosphaera sedula to Thermal and Nutritional Stresses. Appl Environ Microbiol. 1995;61(6):2314–2321. doi: 10.1128/aem.61.6.2314-2321.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett RS. Structure and catalytic mechanism of the β-carbonic anhydrases. Biochim Biophys Acta (BBA) - Proteins and Proteomics. 2010;1804(2):362–373. doi: 10.1016/j.bbapap.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Appl Environ Microbiol. 2011;77(9):2905–2915. doi: 10.1128/AEM.03034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Systems Biol. 2011;7:539–539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Ferry JG. Prokaryotic carbonic anhydrases. FEMS Microbiol Rev. 2000;24(4):335–366. doi: 10.1111/j.1574-6976.2000.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Smith KS, Jakubzick C, Whittam TS, Ferry JG. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proc Natl Acad Sci USA. 1999;96(26):15184–15189. doi: 10.1073/pnas.96.26.15184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WR, Entcheva P. Biotin in microbes, the genes involved in its biosynthesis, its biochemical role and perspectives for biotechnological production. Appl Microbiol Biotechnol. 2003;61(1):21–31. doi: 10.1007/s00253-002-1186-2. [DOI] [PubMed] [Google Scholar]
- Sueda S, Li Y-Q, Kondo H, Kawarabayasi Y. Substrate specificity of archaeon Sulfolobus tokodaii biotin protein ligase. Biochem Biophys Res Comm. 2006;344(1):155–159. doi: 10.1016/j.bbrc.2006.03.118. [DOI] [PubMed] [Google Scholar]
- Thorgersen MP, Lipscomb GL, Schut GJ, Kelly RM, Adams MWW. Deletion of acetyl-CoA synthetases I and II increases production of 3-hydroxypropionate by the metabolically-engineered hyperthermophile Pyrococcus furiosus. Metab Eng. 2014;22(0):83–88. doi: 10.1016/j.ymben.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem. 1948;176(1):147–154. [PubMed] [Google Scholar]
- Yoon SH, Reiss DJ, Bare JC, Tenenbaum D, Pan M, Slagel J, Moritz RL, Lim S, Hackett M, Menon AL, et al. Parallel evolution of transcriptome architecture during genome reorganization. Genome Res. 2011;21(11):1892–1904. doi: 10.1101/gr.122218.111. [DOI] [PMC free article] [PubMed] [Google Scholar]