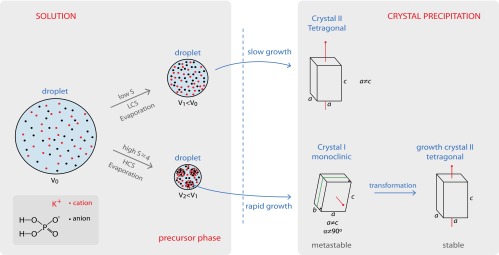
Fig. 1.
Schematic of the crystallization pathways reported by Lee et al. (3). The top path is the “normal” crystallization of the equilibrium crystal shape directly from solution; in the experiments this happens for low concentration solutions (LCS). The lower path happens for high concentrations (HCS): a three-step nucleation process with first the formation of a precursor “phase” consisting of regions that are more concentrated in salt, followed first by the nucleation of a metastable monoclinic crystal before the equilibrium tetragonal crystal appears. S, supersaturation; V, droplet volume.