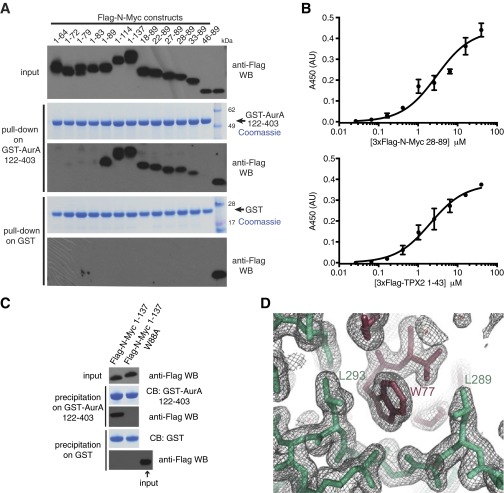
Fig. S1.
Interaction between N-Myc and Aurora-A. (A) Coprecipitation of fragments of the N-Myc transactivation domain with the GST–Aurora-A kinase domain. Flag-tagged N-Myc fragments were incubated with GST–Aurora-A 122–403 D274N or GST alone immobilized on glutathione Sepharose beads. Coprecipitated N-Myc fragments were visualized by anti-Flag Western blot (WB). (B) Binding isotherms measured by ELISA quantifying the interaction of 3xFlag-N-Myc 28–89 (Upper) and 3xFlag-N-Myc TPX2 1–43 (Lower) with immobilized biotinyl Avi-tagged–Aurora-A 122–403. Data points represent the mean of three experiments; error bars indicate SD. (Affinities of 2.9 ± 0.5 μM and 2.0 ± 0.3 μM were measured for 3xFlag-N-Myc 28–89 and 3xFlag-N-Myc TPX2 1–43, respectively.) (C) The specific interaction between the GST–Aurora-A catalytic domain and Flag-tagged N-Myc TAD is abrogated by a single point mutation (Trp88). Glutathione resin precipitates were separated by SDS/PAGE followed by Western blot analysis and Coomassie Blue (CB) staining. (D) A representative region of the electron density map of the 1.72-Å crystal structure of the Aurora-A/N-Myc complex showing the interaction of Trp77 of N-Myc (dark red) with a hydrophobic pocket on Aurora-A (pale green). The mesh represents a 2mFo-DFc map contoured at 1.0 σ.