Significance
Intergroup conflicts are among the world’s most imminent problems, particularly with the shift of battlefields into the heart of civilian locations and the participation of increasingly younger adolescents in intergroup conflict. We found that Israeli and Palestinian adolescents reared in a climate of long-standing strife shut down the brain’s automatic response to outgroup pain. This neural modulation characterized a top-down process superimposed upon an automatic response to the pain of all and was sensitive to hostile behavior toward outgroup, uncompromising worldviews, and brain-to-brain synchrony among group members. Findings pinpoint adolescents’ sociocognitive top-down processes as targets for intervention.
Keywords: intergroup conflict, empathy, alpha oscillations, oxytocin, brain-to-brain synchrony
Abstract
Adolescents’ participation in intergroup conflicts comprises an imminent global risk, and understanding its neural underpinnings may open new perspectives. We assessed Jewish-Israeli and Arab-Palestinian adolescents for brain response to the pain of ingroup/outgroup protagonists using magnetoencephalography (MEG), one-on-one positive and conflictual interactions with an outgroup member, attitudes toward the regional conflict, and oxytocin levels. A neural marker of ingroup bias emerged, expressed via alpha modulations in the somatosensory cortex (S1) that characterized an automatic response to the pain of all protagonists followed by rebound/enhancement to ingroup pain only. Adolescents’ hostile social interactions with outgroup members and uncompromising attitudes toward the conflict influenced this neural marker. Furthermore, higher oxytocin levels in the Jewish-Israeli majority and tighter brain-to-brain synchrony among group members in the Arab-Palestinian minority enhanced the neural ingroup bias. Findings suggest that in cases of intractable intergroup conflict, top-down control mechanisms may block the brain’s evolutionary-ancient resonance to outgroup pain, pinpointing adolescents’ interpersonal and sociocognitive processes as potential targets for intervention.
Intergroup conflicts—among races, religions, cultures, and nations—are one of the world’s most imminent problems, particularly with the shift of battlefields into the heart of civilian locations and the participation of increasingly younger adolescents in intergroup conflict. According to the 2015 World Economic Forum, intergroup conflicts comprise the greatest global risk in the foreseeable future (1). However, how can humans, who evolved as a highly social species and whose brain automatically responds to the pain of others, inflict such pain on their fellow human beings? Here, we attempt to address this ancient question from a unique angle, asking whether neuroscience can offer new insights into the mechanisms that enable humans to tolerate the pain imposed on others. Because the success and thriving of our species depends on the capacity to quickly form social groups and instantly distinguish friend from foe (2), we ask whether our brain already processes the pain of our ingroup and that of the outgroup differently at the automatic level or whether higher-order evaluative processes are superimposed upon a uniform brain response to differentiate “us” from “them.” That is, we ask whether the “ingroup bias” stems from bottom-up or top-down mechanisms and whether this bias can be predicted by endogenous oxytocin (OT) levels, which are known to play a causal role in regulating intergroup relations (3).
The most evolutionary-ancient precursor of empathy involves emotional arousal/resonance to the distress of conspecifics, expressed as simple physiological mirroring in rodents (4) and more broadly in primates (5). Such rudimentary empathy is observed primarily in the nociceptive mechanism (i.e., pain perception), which promotes responsiveness to one's offspring and social group, thus conferring survival advantage. It appears that evolution has tailored pain perception into the mammalian brain as a basic mechanism for social affiliation, ranging from primitive reward and homeostatic processes of pain sensitivity to the most advanced forms of human compassion and extended caregiving (6). Substantial human neuroimaging research has demonstrated the key role of the somatosensory cortex (S1) in pain empathy via modulations of alpha oscillations, termed “mu” rhythm when originating in S1 and possibly implicating mirror-like mechanisms (7–9). Alpha oscillations are suppressed at the immediate poststimulus time window and then rebound and enhance power compared with baseline in response to both the experience of pain in self and observation of pain in others (10). Such early suppression occurs automatically and is unaffected by attentional demands, whereas the later rebound is modulated by cognitive-regulatory mechanisms (11). Hence, alpha oscillations may integrate quick automatic responses with slower top-down mechanisms for processing vicarious pain empathy. When individuals observe pain to ingroup and outgroup members, empathic resonance in S1 shows group-specific activations (12–14); yet, the time course of such differential responses is unknown, nor is information available as to whether these responses express shared initial activations that diverge at evaluative stages (top-down) or a shutdown of even the most basic automatic response to vicarious pain (bottom-up). This important issue taps an age-old question about human beings’ innate nature: How deep is our animosity for those unlike us compared with our compassion for human suffering?
The Israeli–Palestinian conflict is among the most intractable intergroup conflicts worldwide, generating aggression and suffering for over a century, thus providing ecologically valid context for investigation (15). Recently, adolescents’ involvement in this conflict has increased at alarming rates, paralleling the global epidemic of adolescents’ participation and recruitment into conflict via social media; hence, the present focus on Jewish-Israeli and Arab-Palestinian adolescents is timely and relevant. Despite pioneering behavioral (16) and fMRI (17, 18) work on empathic attitudes in the context of the Israeli-Palestinian conflict, comprehensive understanding of the mechanisms via which conflict impedes empathy for others’ suffering is lacking. Moreover, it remains unknown how the neural markers of empathy relate to adolescents’ dialog styles in interpersonal situations and their attitudes toward the intergroup conflict. We also addressed the implications of the ancient OT system on modulations in neural responses to ingroup or outgroup’s pain. Animal studies and human OT administration research have shown that OT increases ingroup affiliation (19), and yet, under conditions of threat it also prepares for defensive aggression toward outgroup targets (3). OT administration was found to increase ingroup bias of the brain’s empathic response and this bias was linked with positive implicit attitudes toward ingroup members (20). Whereas studies mainly tested the effect of OT administration on ingroup bias, the role of endogenous OT has been largely ignored. Here, we tested whether endogenous OT could predict the brain’s empathic response within the intergroup context.
To investigate the neural marker for ingroup bias in pain resonance and its interactional, attitudinal, and neuroendocrine correlates, we recruited Jewish-Israeli and Arab-Palestinian adolescents (N = 80), representing the majority and main minority groups, respectively, in Israel (SI Methods). We first sought to pinpoint a neural marker of pain empathy, reflecting the time course of the brain’s empathic resonance with others’ pain, by using magnetoencephalography (MEG). MEG integrates excellent temporal resolution with good spatial localization and is thus uniquely suited for probing oscillatory dynamics in targeted cortical areas. We used MEG to probe alpha oscillations and their neural source while empathizing with vicarious pain. We then hypothesized that priming of group membership of the target protagonist may bias either early or later neural signature, reflecting bottom-up cascade or top-down regulatory input. Finally, to examine correlates of these neural patterns, we assessed behavioral hostility and empathy during interactions with an outgroup member, attitude of compromise toward the intergroup conflict, and peripheral levels of OT measured at baseline and before and after social interactions.
SI Methods
Subjects’ Demographic Information.
Subjects were residents in the center of Israel (within a 50 km distance from Tel Aviv) and included 40 Arab-Palestinian citizens of Israel (20 males) and 40 Jewish-Israelis (22 males). Similar to the proportions within the general population (43), levels of religiousness in the Arab-Palestinian group was mainly religious Muslim (81% self-reported being religious), whereas in the Jewish-Israeli group, it was mainly secular Jewish (11% self-reported being religious).
The Arab-Palestinian minority group in Israel makes up 21% of the country’s population (44). However, these citizens are Arab-Palestinian by heritage and religion and share a Palestinian collective national identity, which is perceived as a threat by many Jewish-Israelis (45). Jewish-Israeli and Arab-Palestinian adolescents typically study in separate schools and live in different neighborhoods or towns. In the vast majority of cases, adolescents from the two groups never have opportunities for interpersonal encounters.
Stimuli During MEG Brain Activity Measurement.
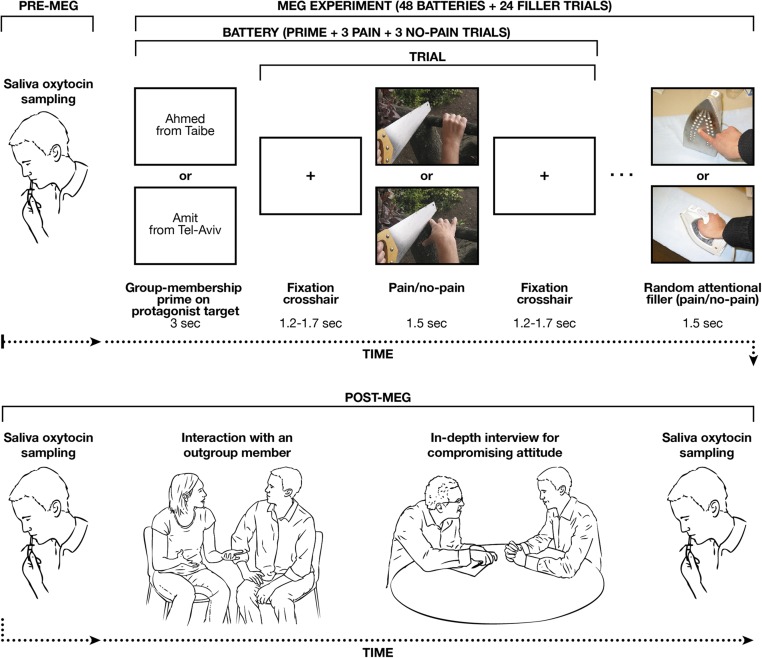
The MEG-measured experiment included three types of stimuli: all stimuli appeared in uniform size (300 × 225 pixels) at the center of a gray background on a 20-inch monitor, subtending a visual angle of 20.96° × 15.37° at a viewing distance of 50 cm. A series of 96 digital color pictures showed limbs (right hands and right feet) in P (48 stimuli) and no-P (48 stimuli), at a ratio of 51/49% for legs/hands (Fig. 1). The series was repeated three times at a pseudorandom order with eight batteries of six randomly distributed trials (three P and three no-P), summing up to 288 trials in total. Each battery depicted a protagonist whose limbs were shown. The P stimuli and no-P stimuli underwent previous validation (46) and are most commonly used in many behavioral and neuroimaging studies. All 48 P-stimuli pictures depicted familiar events that can happen in everyday life involving mechanical, thermal, and pressure pain. The 48 no-P pictures involved the same settings without any painful component. 24 filler trials were used.
Fig. 1.
Experimental procedures are depicted with the upper panel showing the pre-MEG experiment sampling of saliva OT and then the course of the MEG experimental session (N = 80). Lower shows the post-MEG procedures (saliva OT sampling, outgroup interaction and in-depth interview for compromising attitude).
The procedure included priming (before each battery) of the P and no-P pictures for either Jewish-Israeli or Arab-Palestinian group membership of the person whose limbs were shown (Fig. 1). Each priming text screen (24 Arab-Palestinian and 24 Jewish-Israeli screens) included both a first name and a geographic origin, such a Gil from Tel Aviv for Jewish-Israeli priming or Ahmed from Taibe for Arab-Palestinian priming. This explicit ingroup/outgroup priming procedure presents the protagonist’s name and residence, consistent with previous research (16). The eight (repeated three times) Arab-Palestinian target names (Ibrahim, Rashid, Shahad, Ahmed, Chasan, Mustafa, Fatma, Ali) and the eight (repeated three times) Jewish-Israeli target names (Ronit, Arie, Amit, Dan, Gil, Ayelet, Yosi, Eran) used as primes in the current experiment are very common Arab-Palestinian and Jewish-Israeli names. The eight Arab-Palestinian target geographical locations (Baka-Al-Garabiya, Jaffa, Sachnin, Kfar Kasem, Jaljulya, Kfar Yasif, Daburiya, Taibe) and the eight Jewish-Israeli target geographical locations (Kfar Saba, Yahud-Monoson, Zichron Yaakov, Kiryat Tivon, Rishon leTsiyon, Tel Aviv, Tiberias, Petach Tikva) used as primes in the current experiment are famous cities or towns in Israel known for either their Jewish-Israeli or Arab-Palestinian majority. The priming stimuli all underwent validation for group membership by 10 independent Arab-Palestinian and Jewish-Israeli raters.
In addition, we used attentional filler stimuli (Fig. 1) as fillers to avoid participants’ habituation or anticipation and to maintain a steady alertness level throughout the experiment. These stimuli were P and no-P pictures presented with a Photoshop twirl filter (Adobe Systems).
MEG Data Cleaning.
Four steps aimed to clean artifacts and noise: (i) we removed external noise (e.g., power-line, mechanical vibrations) and heartbeat artifacts from the data using a predesigned algorithm for that purpose; (ii) we rejected trials containing muscle artifacts using visual inspection; (iii) we removed eye blinks, eye movements, or any other potential noisy artifacts using spatial component analysis (ICA); and (iv) a final visual inspection of every trial verified any other noise/artifact to be removed from further analysis. We excluded one sensor from the analysis due to malfunction. We filtered the data in the 1- to 200-Hz range with 10-s padding and then resampled them to 400 Hz.
Head Grid and Source Reconstruction.
We divided the subject’s brain volume into a regular grid, obtaining the grid positions by their linear transformation in a canonical 1-cm grid. This procedure facilitates group analysis, because it requires no spatial interpolation of the volumes on reconstructed activity. For each grid position, we reconstructed spatial filters in the aim of optimally passing activity from the location of interest, while suppressing activity that was not of interest. We computed the cross-spectral density matrix between all MEG sensor pairs from the Fourier transforms of the tapered data epochs. We constructed spatial filters for each grid location, based on the identified frequency bin, and projected the Fourier transforms of the tapered data epochs through the spatial filters. For the ISC analysis, the virtual-channel time series (in 100-ms steps) wherein the ingroup bias was detected across all subject pairs were averaged. This process was performed similarly to a recent ISC study in MEG and EEG (47). The resulting index per subject should reflect the degree of neural synchrony (related to the ingroup-bias) with the remaining participants. We assumed that a high degree of synchrony of the neural ingroup-bias response should reflect higher cohesion with the remaining ingroup members.
The Nonparametric Statistical Approach.
This nonparametric permutation approach does take the cross-subject variance into account, because this variance is the basis for the width of the randomization distribution. This approach is valuable because it does not make any assumptions about underlying distribution and is unaffected by partial dependence between neighboring time–frequency pixels. Specifically, in the first step of the procedure, we computed t values representing the contrast between the conditions. Subsequently, we defined the test statistic by pooling the t values over all participants. Here, we searched clusters with effects that were significant at the random effects level after correcting for multiple comparisons. To compute the effect compared with baseline, the first step was replaced by adjusting the effect to the baseline level, and the second step applied a dependent t test. These procedures would correspond to fixed-effect statistics, however, to make statistical inferences corresponding to a random effect statistic, we tested the significance of this group-level statistic by means of a randomization procedure: we randomly multiplied each individual t value by 1 or by −1 and summed it over participants. Multiplying the individual t value with 1 or −1 corresponds to permuting the original conditions in that subject.
We reiterated this random procedure 1,000 times to obtain the randomization distribution for the group-level statistic. For each randomization, we retained only the maximal and the minimal cluster-level test statistic across all clusters, placing them into two histograms that we addressed as maximum/minimum cluster-level test statistic histograms. We then determined, for each cluster from the observed data, the fraction of the maximum/minimum cluster-level test statistic histogram that was greater/smaller than the cluster-level test statistic from the observed cluster. We retained the smaller of the two fractions and divided it by 1,000, giving the multiple comparisons corrected significance thresholds for a two-sided test. The proportion of values in the randomization distribution exceeding the test statistic defines the Monte Carlo significance probability, which is also called a P value (41). This cluster-based procedure allowed us to obtain a correction for multiple comparisons in all brain analyses.
Dyadic Outgroup Interaction Procedure.
For the positive dialog, we gave the dyad 10 min to plan a “fun day” to spend together. For the conflict dialog, we asked the same dyad to choose any conflict and discuss it for 10 min, whether related to their personal life (e.g., with parents, teachers) or to the national conflict. Interactions then underwent coding offline by coders blind to any other information using the well-validated Coding Interactive Behavior (CIB) manual (48). Interrater intraclass reliability computed on 20 positive and 20 conflict interactions averaged r = 0.93 (range: 0.88–0.99). We computed two coding composites, Empathy and Hostility, by averaging several CIB codes in each paradigm and then averaging them across both paradigms, consistent with previous research (42). The Empathy composite included the following codes: empathy, positive affect, elaboration, praising, dyadic reciprocity, and synchrony. The Hostility composite included the following codes: hostility, withdrawal, intrusiveness, and assertiveness. These constructs indexed the degree to which each participant exhibited empathy or hostility toward the outgroup member during each of the one-on-one social interactions.
Interview Procedure Tapping Compromising Attitudes Toward Intergroup Conflict.
The interview included 43 topics; in brief, the topics covered possible solutions for the conflict, how one should conduct dialog about the conflict both within personal relationships and among nations, what Jews should do to improve the situation, what Arabs should do to improve the situation, and who is to blame. Interviewers rated the participants’ attitude toward each item on a three-point scale ranging from 1 (strong opposition) to 3 (full endorsement). We then computed a measure of a Compromise attitude by averaging seven items that describe the degree to which participants endorsed compromise as the main solution for the conflict. This construct included group-specific and general items. The group-specific items for Jewish-Israelis included “endorsement of a two-state solution” and “recognition of the Arab-Palestinians’ suffering during establishment of the state.” For the Arab-Palestinians, group-specific items included “willingness to accept the existence of Jews in Israel,” “letting go of the idea of a Palestinian-only land,” and “stopping the rhetoric of hatred and acts of aggression toward Jewish-Israelis.” General items included “meetings with the other group should be encouraged” and “there is no absolute justice.”
Results
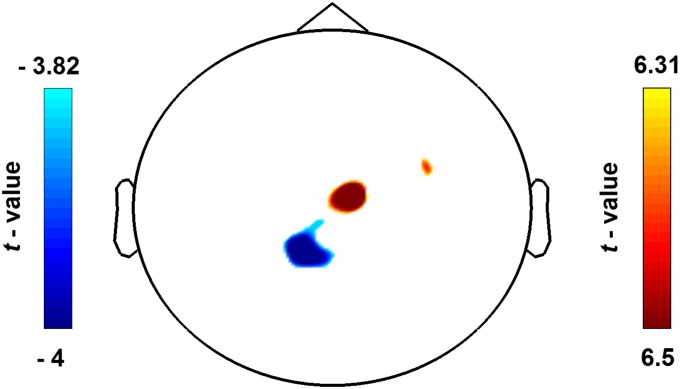
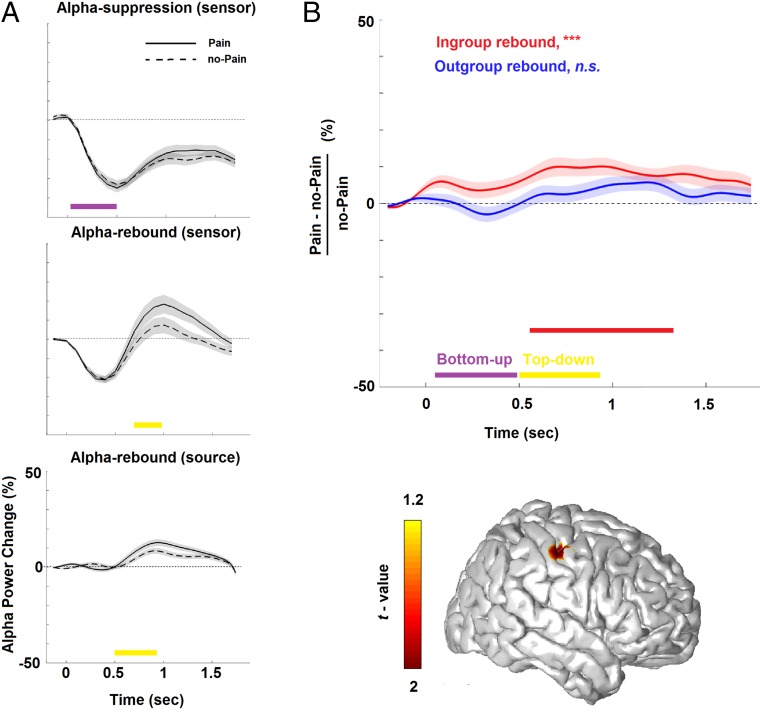
Adolescents watched a set of well-validated visual stimuli depicting limbs in painful or nonpainful conditions (14), preceded by a prime-linking stimuli to either an Arab-Palestinian or Jewish-Israeli protagonist (in total four within-subject conditions), while we measured ongoing oscillatory neural activity using MEG (Fig. 1). The detection rate in the attentional filler task (Fig. 1) was high (mean ± SD, 93.05 ± 8.58%). As expected, the MEG sensor-array detected that the neural response to Pain (P) and to no-Pain (no-P) stimuli was expressed above central sensors (Fig. S1) as alpha (7- to 11-Hz) suppression (descent to suppression peak at ∼50–500 ms), presumably mirroring bottom-up processing (purple rectangle) (Fig. 2A, Upper); it was then followed by alpha (9- to 15-Hz) rebound (ascent to rebound peak at ∼700–950 ms), presumably mirroring top-down processing (yellow rectangle) (Fig. 2A, Middle). We then proceeded to localizing the neural substrates characterizing pain empathy (P vs. no-P). Alpha enhancement was localized (Pcluster-cor < 0.05) primarily in the right sensorimotor cortex (S1) (in BA3); yet, no significant source emerged for the early alpha suppression (Pcluster-cor > 0.70), suggesting that the sample of 80 adolescents consistently revealed the main effect of pain empathy (i.e., P compared with no-P) through the alpha rebound in the right S1 (Fig. 2B, Lower), with ascent to rebound peak at ∼500–920 ms (Fig. 2A, Lower).
Fig. S1.
Scalp topographies illustrate the P vs. no-P contrast, resulting in induced suppression as shown in blue and alpha rebound as shown in red. Color bars illustrate masked statistical significance (Pcluster-cor < 0.01).
Fig. 2.
Alpha power change in response to vicarious pain (N = 80). (A) Plots of the temporal evolution of alpha-band–induced power change (normalized to baseline activity) in response to P and no-P stimuli. (B) Alpha rebound in the somatosensory cortex (see peak activity in the bottom panel illustrating the overlaid cortical surface) for pain empathy (P/no-P ratio) of ingroup (red) and outgroup (blue) protagonists. Shades represent ±1 SEM. Rectangles describe descent to peak suppression (purple) and ascent to peak rebound (yellow), thereby, respectively, mirroring bottom-up and top-down processes. Red rectangle describes statistically (cluster-based statistics) significant effect (***Pcluster-cor < 0.001) on the time axis. The color bar illustrates masked statistical significance (Pcluster-cor < 0.05).
A Top-Down Neural Ingroup Bias.
To examine whether priming of protagonists’ group membership bias (i.e., pain of ingroup vs. outgroup) taps top-down processing, a repeated-measures ANOVA examined group bias (Arab-Palestinian/Jewish-Israeli) and stimulus bias (ingroup/outgroup) effects in S1 (ratio of P/no-P). A significant main effect emerged for ingroup/outgroup stimulus bias (Pcluster-cor < 0.005), but no significant group or interaction effects emerged between the Jewish-Israeli and the Arab-Palestinian adolescents; that is, adolescents of both nationality responded differently to pain of ingroup and outgroup protagonists. Fig. 2B, Upper illustrates the pain empathy effect (P/no-P ratio in S1), which was biased by the protagonists’ group membership. As seen in the figure, the expected significant enhancement of rebound from baseline in response to protagonists’ pain (P vs. no-P) occurred only toward the ingroup target (540–1,360 ms, Pcluster-cor < 0.001) and clearly occurred within the range of top-down processing (see red rectangle in Fig. 2B, Upper); there was no P vs. no-P effect when priming was toward the outgroup target stimuli (no clusters). These findings suggest that group membership of the protagonist who is experiencing the pain strongly biases alpha oscillations’ late rebound, such that they occur only toward ingroup protagonists and not at all toward outgroup protagonists. Notably, no significant difference emerged in the early component of the alpha oscillations, the sensor-level alpha suppression, toward ingroup versus outgroup protagonists (P > 0.8).
Brain-to-Brain Synchrony.
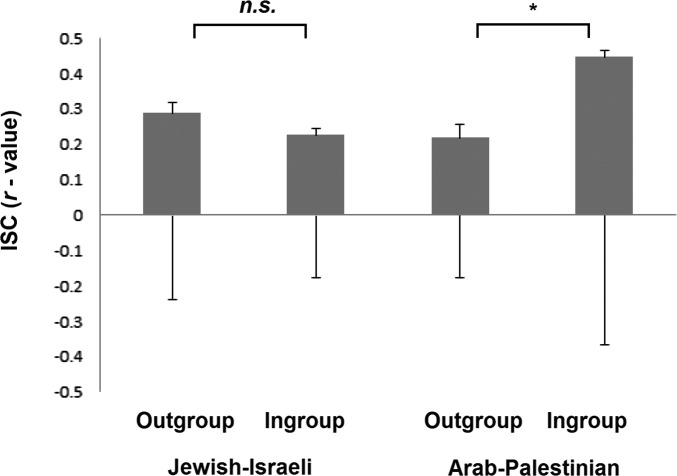
Once we identified a neural marker in S1 for ingroup bias in pain resonance in both Jewish-Israeli and Arab-Palestinian adolescents, we explored how this ingroup bias may relate to group cohesion at a neural level. Brain-to-brain synchrony was measured using the intersubject correlation (ISC) index (SI Methods). Repeated-measures ANOVA yielded a significant demographic background by ingroup-bias interaction effect [F(1,78) = 5.10, P = 0.02] but no significant effects for ingroup bias [F(1,78) = 1.72, P = 0.19 or demographic-background F(1,78) = 2.16, P = 0.14]. Post hoc t tests revealed that Arab-Palestinian adolescents showed significantly higher ISC when protagonists were members of their ingroup (mean = 9.6, SD = 24.71) than when the protagonists were outgroup members [mean = 0.25, SD = 11.55; t(39) = 2.25, P = 0.03]. The Jewish-Israelis showed no such ISC difference [t(39) = −0.77, P = 0.44 (Fig. S2)]. In line with this finding, an ethnocentricity questionnaire revealed that Arab-Palestinian adolescents reported greater ethnocentricity compared with Jewish-Israeli adolescents [t(73) = −4.15, P < 0.0001].
Fig. S2.
ISC values for Jewish-Israeli participants and Arab-Palestinian participants while attending to ingroup and outgroup target stimuli. Error bars represent ±1 SEM. Asterisks describe statistical significance (*P < 0.05).
The Neural Ingroup Bias Is Related to Social Behavior, Attitudes Toward Conflict, and Oxytocin.
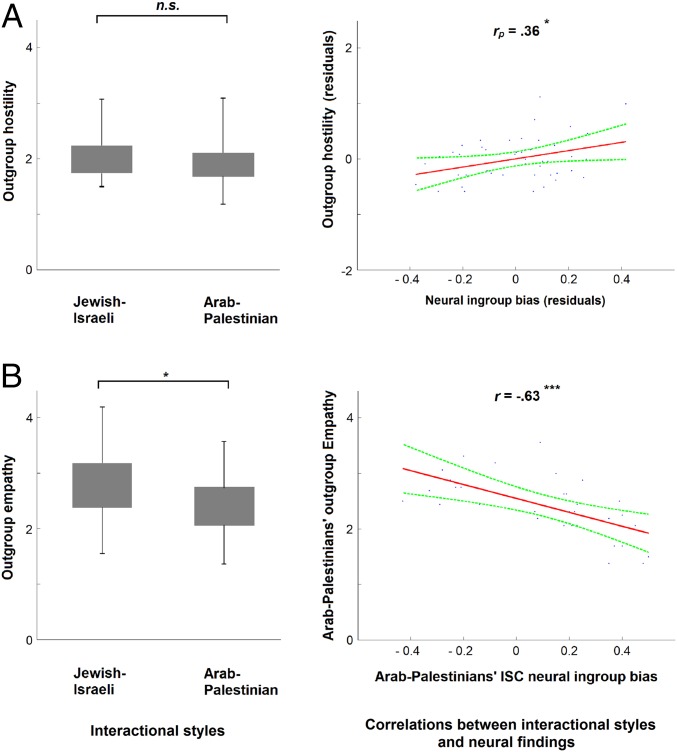
Having identified this neural marker of ingroup-bias in S1, along with the synchronized ISC ingroup bias for the Arab-Palestinians, we next examined its behavioral, cognitive, and neuroendocrine correlates. We first observed adolescents’ social behavior toward an outgroup member in two one-on-one interactions: a “conflict dialog” where the dyad negotiated a conflict of their choice and a “positive dialog” where the dyad planned a fun day (SI Methods). Next, using an in-depth interview to tap attitudes toward the intergroup conflict, we measured the degree to which adolescents perceived Compromise as the path for resolving conflicts in general, and the Israeli-Palestinian conflict in particular (SI Methods). The two groups revealed a medium-low level (on a scale of 1 to 5: mean = 1.98, SD = 0.37) of intergroup hostility (Fig. 3A, Left) during actual interactions and expressed a rather low level (on a scale of 1 to 3: mean = 1.30, SD = 0.21) of willingness for intergroup compromise, with no significant difference between the two nationalities on these two measures (P > 0.15). By contrast, the Arab-Palestinians showed less [t(58) = −2.45, P = 0.01] empathy (on a scale of 1 to 5: mean = 2.41, SD = 0.53) toward the outgroup member than did Jewish-Israelis (on a scale of 1 to 5: mean = 2.78, SD = 0.62) (Fig. 3B, Left).
Fig. 3.
Relations between neural ingroup-bias and interactional behavior during dyadic interactions. (A) Groups’ hostility (N = 67) scores (Left) and partial pairwise correlation (rp) with both groups’ dyadic (N = 50) neural ingroup-bias (Right). (B) Groups’ empathy (N = 60) scores (Left) and the correlation (Pearson’s r) of the Arab-Palestinian scores (N = 32) with their ISC neural scores (Right). Error bars represent ±1 SEM. Asterisks describe statistically significant (independent t tests) effect (*P < 0.05; **P < 0.005; ***P < 0.0005).
We next examined whether the neural marker of ingroup bias can be predicted by hostile social behavior toward outgroup or by low scores on compromise. Given that hostility levels were similar across groups, we examined whether it would predict individual differences in the neural ingroup bias for the entire sample. As expected (Fig. 3A, Right), the neural ingroup bias was explained by increased hostility during interaction with outgroup members (rp = 0.36, P = 0.01) and by lack of compromise in the context of the conflict (r = −0.37, P = 0.002), whereas no significant correlation emerged for behavioral empathy (rp = −0.11, P = 0.50).
Arab-Palestinians expressed less empathic behavior toward their Jewish peers than vice versa; thus, we measured whether this finding can explain their greater brain-to-brain cohesion (ISC scores) toward ingroup targets. Brain-to-brain synchrony (ISC scores) to the pain of ingroup protagonists target stimuli did not significantly correlate with behavioral empathy (rp = −0.21, P = 0.17) or with hostility (rp =0.20, P = 0.16). Because group scores in both brain-to-brain synchrony and behavioral empathy significantly differed, we looked at the association between behavioral empathy and brain-to-brain synchrony within each group. We found that the two variables were significantly correlated in the Arab-Palestinian group (r = −0.63, P = 0.0001) (Fig. 3B, Right) but not in the Jewish-Israeli group (r =0.03, P = 0.86).
Finally, the OT system develops in the context of mammalian parenting and is highly sensitive to variability in maternal touch, contact, and behavioral synchrony (2, 21). Parent–infant interactions in Jewish-Israeli and Arab-Palestinian societies show markedly different patterns, particularly in the amount of touch (higher in Arab-Palestinians) and behavioral synchrony (higher in Jewish-Israelis) (22). We thus examined OT levels and its covariation with neural ingroup bias for each group separately. For Jewish-Israeli participants, OT levels linearly increased with the extent of the neural ingroup bias (r = 0.32, P < 0.05), corroborating a previous report on the tight link between ingroup bias and OT (19); nevertheless, there was no link between ingroup-bias and OT levels for the Arab-Palestinian participants (r = −0.03, P = 0.84).
Discussion
At least one-fifth of humanity lives in regions of the world experiencing significant violence, political conflict, and chronic insecurity. Following the recent call in social neuroscience to ground investigations in real-life social issues and focus on brain-to-brain mechanisms (23–25), our study examines the neural basis of intergroup conflict by using magnetoencephalography integrated with behavioral, attitudinal, and neuroendocrine measures. Among youth growing up within one of the world’s most intractable conflicts, we identified a neural marker for ingroup bias and pinpointed its oscillatory frequency, temporal course, and cortical generator. Specifically, we found that adolescents shut down their brain response to the pain of outgroup targets while showing the expected alpha rebound to ingroup protagonists in a specific area of the somatosensory cortex (S1), which has been repeatedly shown in both electrophysiology and fMRI studies to activate in response to others’ pain (7–9). Such consistency of S1 recruitment across studies and methods suggests that the S1 source localization described here can be assumed as accurate, despite relying on inverse estimate solution. Importantly, our study targeted the adolescent brain, which is considered a brain in transition whose development marks a shift from visceral-emotional to more evaluative processing (26). It would be relevant for future studies to test how responses to ingroup versus outgroup develop from childhood to adulthood. One possibility is that the more developed evaluative function in adults would attenuate the ingroup bias; alternatively, the higher brain plasticity in children and adolescents may lead to more pronounced bias in adulthood.
Consistent with prior research, vicarious pain empathy was expressed via modulations of alpha oscillations (7, 9), suggesting that up- and down-regulation of mirror-like mechanisms may be implicated in the human capacity to empathize with, as well as walk away from the pain inflicted on others. Importantly, this differential alpha response in S1 characterized a top-down process, observed at 540–1,360 ms poststimulus that followed a uniform automatic response to the pain of all, indicating that sociocognitive processes are superimposed upon an evolutionary-ancient response to human suffering to differentiate friend from foe. Interestingly, previous work showed that ipsilateral alpha power increases to suppress distracting input (27). In the context of the current experiment, it may suggest that participants’ (right-hemispheric) brain response to right-sided limbs reflected S1 disengagement. Finally, individual differences in hostile behavior toward outgroup during one-on-one encounters and uncompromising attitudes toward the conflict enhanced the neural marker. Thus, our findings have clear translational relevance and indicate that opportunities for personal contact with outgroup members and respect for multiple worldviews may chart one avenue for youth interventions based on neuroscience insights.
Mechanisms that enable humans to understand the emotions and actions of others function through online crosstalk between bottom-up and top-down processes, fast sensory–motor integration and slower sociocognitive predictions (23, 28), with specific dynamics defining distinct end products. Top-down processes are shaped by prior learning, attentional demands, regulatory abilities, and social goals, and authors have suggested that brain oscillations provide a useful vantage-point to tap the balance of bottom-up automaticity and top-down-regulation in understanding social phenomena (21). Human vicarious pain empathy integrates evolutionary-ancient automaticity with higher-order regulation; thus, understanding its neural underpinnings requires attention to both and such integration has rarely been examined in human research. Our study—which tests vicarious pain empathy using MEG while integrating social behaviors, interviews, and hormones—provides a unique example for how the balance of fast and slow processing may address critical questions in social neuroscience that cannot be answered by other tools (e.g., fMRI). The findings that both Jewish-Israeli and Arab-Palestinian youth exhibited the same bottom-up activation to ingroup member and the same top-down attenuation to outgroup member may suggest that we have detected a universal mechanism whose correlates may differ across cultures, but its core components remain constant.
Brain-to-brain synchrony and OT showed culture-specific associations with the neural ingroup bias; brain-to-brain synchrony was associated with increased ingroup bias among Arab-Palestinians and higher OT correlated with greater bias in the Jewish-Israeli group. Even low ISC values in electromagnetic recordings strongly predict heightened attention (29) and preference (30). This finding is suggestive of brain-to-brain synchrony among Arab-Palestinians to reflect preference to attend to the suffering of their group members. Brain-to-brain synchrony is also suggested to underlie shared psychological experiences and to bind members of a group into a collective unit (31). This interpretation fits well with the minority status of Arab-Palestinians and accords with the survival function of such group-binding mechanism to enhance group cohesion in the face of external threats (32). Possibly, in more collectivistic societies and in minority groups that feel a threat to group identity, this mechanism is more active, as seen in our findings, and may reflect an often-observed strategy of minority groups to gain power by acting collectively (33). Because social cooperation differs by social status (33), the difference between groups in brain-to-brain synchrony may relate to the social status differences between Arab-Palestinians and Jewish-Israelis. At the same time, our results demonstrate the downside of such group-binding mechanism; the greater the ISC index of Arab-Palestinian adolescents, indicating greater neural binding to the group, the lower was their behavioral empathy to outgroup member, suggesting that in such contexts brain-to-brain synchrony may be a mechanism to cope with disempowerment perhaps by excluding the outgroup majority (34). Indeed, Arab-Palestinian adolescents reported greater ethnocentricity compared with Jewish-Israeli adolescents, and the collectivistic schema may have shaped the ingroup-bias at the neural level, consistent with recent findings in a priming experiment (35).
OT functioned in the same way in the Jewish-Israeli group. Whereas higher peripheral OT has been linked with social collaboration, trust, and generosity, research has also implicated OT in ingroup love and outgroup derogation, particularly when the ingroup experiences threat from the outgroup (3). Throughout animal evolution, the ancient OT molecule, which presumably evolved ∼600 million years ago via gene duplication in jawed fish, enabled organisms to adapt to harsh ecologies by forming social collaboration but also by refining differentiation of ingroup from outgroup members (36). The present findings may be interpreted in the context of the Israeli-Palestinian conflict. Because violence is often experienced between Israeli officials (i.e., police, military) and Arab-Palestinian adolescents, Jewish-Israeli adolescents may see Arab-Palestinian adolescents as a direct threat, rather than vice versa. Hence, outgroup threat experienced by Jewish-Israeli adolescents may trigger the OT system. Future studies should further probe these interesting speculations on the various biological mechanisms (i.e., brain-to-brain synchrony and OT) that bind groups together while at the same time sustain the ingroup bias.
In sum, our findings offer a perspective on the global epidemic of adolescents’ exposure to intractable conflict by testing the neural underpinning of the ingroup bias and its temporal dynamics. We detected a neural marker for the adolescent brain’s differential response to the pain of a person in their own ingroup versus someone who is in the outgroup with whom they are in intractable conflict. We demonstrated that youngsters who grow up in a climate of long-standing intergroup strife shut down the brain’s automatic response to the pain of outgroup members through a late and sustained rhythmic top-down mechanism for processing vicarious pain empathy. We further showed that behavioral hostility and unwillingness for intergroup compromise explain this ingroup-bias. Dehumanization of outgroup members was underpinned by unique neural processes in each group: increased brain-to-brain synchrony in the more collectivistic Arab-Palestinian minority society and increased functioning of the oxytocinergic system in the more individualistic Jewish-Israeli majority. Because the brain’s top-down control mechanisms develop on the basis of prior experience and are highly sensitive to social construals, education, and propaganda, our findings pinpoint targets for youth interventions that may promote compassion at the neural level: provision of opportunities for one-on-one encounters with outgroup members, helping adolescents understand the sociopolitical value of compromise and adult modeling on how to conduct dialog with respect and empathy.
Methods
Subjects.
Eighty-five healthy human adolescents were recruited for this study via social media, advertisement in schools, and in adolescents’ organizations. Inclusion criteria were defined so that participants were right-handed, without history of neurological or psychiatric disorders, wore no metallic items (which could not be removed before the experiment) and whose head did not deviate from the initial position in the MEG helmet. Five of the participants were excluded: two participants did not complete the experiment (reported unbearable pain staying in the MEG without movement), one constantly coughed and moved, one moved excessively (deviation of more than 2 cm), and one moved more moderately (deviation of ∼1 cm) but was still excluded to match the two groups’ sample size. Hence, a final cohort of 80 adolescent high school students (50% Arabs-Palestinians; 52.5% males; age: 15.5–18.5 y, mean ± SD, 16.63 ± 0.89 y). The study received approval from the Bar-Ilan University ethics committee, and participants gave written informed consent before the experiment in line with Bar-Ilan University’s Institutional Review Board. Subjects received monetary compensation for their participation. See SI Methods for further demographic information on the subjects.
Experimental Procedure.
Participants lay in supine position inside the MEG system while facing a screen projecting the stimuli. Subjects received instructions to remain relaxed and not move their limbs; the experimenter observed their compliance using an infrared camera. We programmed and operated the experiment using E-Prime software (Psychology Software Tools). We presented all words and experimental instructions in the participant’s mother tongue (either Hebrew or Arabic).
We used four conditions: ingroup P, ingroup no-P, outgroup P, and outgroup no-P. The purpose of pain (P) stimuli was to elicit empathy, whereas that of no-pain (no-P) stimuli was to not elicit empathy but to control for the other parameters induced by the visual stimuli; filler stimuli were used to maintain attention throughout the experiment (Fig. 1). See SI Methods for more information on the stimuli used.
The stimuli presented while measuring participants’ brain activity comprised a total of 288 trials, grouped into 48 batteries of 6 trials each (3 P and 3 no-P trials). We counterbalanced the order of the six-trial series and the pictures assigned to the protagonist targets across participants, to avoid unspecific stimulus or structure effects. Every six-trial series began with explicit priming for 3 s on the group membership of the Arab-Palestinian or Jewish-Israeli protagonist whose limbs would be presented over the next six screens. Hence, all six of the stimuli in each series (the three P stimuli and the three no-P stimuli) were primed as belonging to the same Jewish-Israeli or Arab-Palestinian individual. P and no-P stimuli were presented for 1.5 s each, interleaved with crosshair fixation screens randomly varying in duration between 1,169 and 1,670 ms (Fig. 1). In addition, filler trials comprised ca. 8% of all trials. The experimenter asked participants to recall and report the occurrences of the filler trials at each pause (every ca. 1.5 min; there were 12 pauses throughout the experiment). We did not include the filler trials in the experimental stimuli database or analyze them.
MEG Recordings and Data Preprocessing.
We recorded ongoing brain activity (sampling rate, 1,017 Hz, online 1- to 400-Hz band-pass filter) using a whole-head 248-channel magnetometer array (Magnes 3600 WH; 4-D Neuroimaging) inside a magnetically shielded room. Reference coils located ∼30 cm above the head, oriented by the x, y, and z axes, enabled removal of environmental noise. See SI Methods for more information on data cleaning. We segmented the data into 1,950-ms epochs, including a baseline period of 470 ms and then filtered it in the 1- to 200-Hz range with 10 s padding and then resampled them to 400 Hz.
Source and Spectral Analyses.
We attached five coils to the participant’s scalp to record head position relative to the sensor. We performed analyses using MATLAB 7 (MathWorks) and the FieldTrip software toolbox (37). We built a single shell brain model based on an MNI postpuberty template brain (38), which we modified to fit each subject’s digitized head shape using SPM8 (Wellcome Department of Imaging Neuroscience, University College London; www.fil.ion.ucl.ac.uk). Head shape underwent manual digitization (Polhemus FASTRAK digitizer). We applied adaptive spatial filtering (39) relying on partial canonical correlations. See SI Methods for more information on head shape model (grid) and source reconstruction.
Finally, we extracted time series from regions of interest by applying a linear constrained minimum variance beam former. We applied tapers to each time window to compute time–frequency representations (TFRs) of power for each trial and to calculate the fast Fourier transform (FFT) for short sliding time windows. We analyzed data in alignment to stimulus onset and then averaged the power estimates across tapers. A Hanning taper, applied to each epoch of the 248-sensor data, yielded the FFT for short sliding time windows of 0.5 s in the broad alpha 7- to 15-Hz frequency range, resulting in a spectral resolution of 2 Hz. We obtained induced activity by subtracting evoked-components’ power from oscillatory power. These time series were also used to calculate ISCs (ISC–Pearson). See SI Methods for more information on the ISC analysis.
Statistical Analysis.
In all statistical comparisons between groups on the behavioral and endocrinal measures, we applied an independent two-sided t test. Correlations between neural and behavioral data for each group applied Pearson’s r, whereas correlations for both groups completed at the dyadic level by applying partial pairwise correlations rp (40). Furthermore, statistical procedures on the MEG data assessed significance of the power values using a randomization procedure (41). See SI Methods for more information on this statistical procedure.
Behavioral and Hormonal Measurements.
To test adolescents’ social behavior toward an outgroup member during one-on-one interactions, after MEG sessions (Fig. 1), we applied two well-validated paradigms, a positive dialog and a conflict dialog (42), between same-sex mixed-group partners, one Jewish-Israeli and one Arab-Palestinian, randomly assigned. To tap views and attitudes regarding the Israeli-Palestinian conflict, we conducted an in-depth structured individual interview with each participant. See SI Methods for information on the dialogs, interview, and coding procedures. Finally, we collected saliva samples using Salivette (Sarstedt) at three time points: upon arrival, after the MEG experiment, and before departure. We kept saliva samples ice-chilled for up to 1 h before centrifuge at 4 °C at 1,500 × g for 15 min and then stored liquid samples at −80 °C. To concentrate the samples by three to four times, we lyophilized liquid samples overnight and kept them at −20 °C until assayed. We reconstructed dry samples in the assay buffer immediately before analysis using the Oxytocin ELISA kit (Assay Design; through ENZO). We performed measurements in duplicate, calculating the concentration of samples using MATLAB 7 (MathWorks) according to relevant standard curves. The intraassay and interassay coefficients were <12.3 and <14.5%, respectively.
Acknowledgments
We thank, in particular, Galit Schneider and Shahar Aberbach for invaluable help in MEG acquisition. We also thank Tal Paz for invaluable help in experimental coordination, Yasmeena Taha for language coordination, Hajar Masarwah for translation of experimental material, Maayan Harel for graphical illustrations, Dafna Lustig for assistance in experimental coordination, and Yuval Harpaz for technical support. We also thank the two anonymous reviewers for their constructive feedback. The work was supported by grants from the Fetzer Foundation, Israel-German Foundation (1114-101.4/2010), the Irving B. Harris Foundation, the Simms-Mann Foundations, and the Israeli Centers of Research Excellence (i-CORE) Program of the Planning and Budgeting Committee and The Israel Science Foundation (Grant 51/11).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612903113/-/DCSupplemental.
References
- 1.World Economic Forum 2015 Global Risks Report (World Economic Forum, Geneva). Available at www3.weforum.org/docs/WEF_Global_Risks_2015_Report15.pdf. Accessed January 1, 2016.
- 2.Feldman R. The adaptive human parental brain: Implications for children’s social development. Trends Neurosci. 2015;38(6):387–399. doi: 10.1016/j.tins.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 3.De Dreu CKW, Kret ME. Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol Psychiatry. 2016;79(3):165–173. doi: 10.1016/j.biopsych.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Burkett JP, Andari E, Curry DC, de Waal FBM, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351(6271):5–9. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser ON, Stahl D, Aureli F. Stress reduction through consolation in chimpanzees. Proc Natl Acad Sci USA. 2008;105(25):8557–8562. doi: 10.1073/pnas.0804141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decety J, Knafo A. 2015. Empathy. Brain Mapping: An Encyclopedic Reference, ed Toga AW (Academic, London), Vol 3, pp 191–194.
- 7.Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci. 2005;8(7):955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- 8.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Yang CY, Lin CP, Lee PL, Decety J. The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. NeuroImage. 2008;40(4):1833–1840. doi: 10.1016/j.neuroimage.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 10.Caetano G, Jousmäki V, Hari R. Actor’s and observer’s primary motor cortices stabilize similarly after seen or heard motor actions. Proc Natl Acad Sci USA. 2007;104(21):9058–9062. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci. 2006;26(2):490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensorimotor resonance with other-race pain. Curr Biol. 2010;20(11):1018–1022. doi: 10.1016/j.cub.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 13.Riečanský I, Paul N, Kölble S, Stieger S, Lamm C. Beta oscillations reveal ethnicity ingroup bias in sensorimotor resonance to pain of others. Soc Cogn Affect Neurosci. 2015;10(7):893–901. doi: 10.1093/scan/nsu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry A, Bentin S, Bartal IB-A, Lamm C, Decety J. “Feeling” the pain of those who are different from us: Modulation of EEG in the mu/alpha range. Cogn Affect Behav Neurosci. 2010;10(4):493–504. doi: 10.3758/CABN.10.4.493. [DOI] [PubMed] [Google Scholar]
- 15.Bar-Tal D. Societal beliefs in times of intractable conflict: The Israeli case. Int J Confl Manage. 1998;9:22–50. [Google Scholar]
- 16.Shamay-Tsoory SG, et al. Giving peace a chance: Oxytocin increases empathy to pain in the context of the Israeli-Palestinian conflict. Psychoneuroendocrinology. 2013;38(12):3139–3144. doi: 10.1016/j.psyneuen.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Bruneau EG, Saxe R. Attitudes towards the outgroup are predicted by activity in the precuneus in Arabs and Israelis. NeuroImage. 2010;52(4):1704–1711. doi: 10.1016/j.neuroimage.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 18.Bruneau EG, Dufour N, Saxe R. Social cognition in members of conflict groups: Behavioural and neural responses in Arabs, Israelis and South Americans to each other’s misfortunes. Philos Trans R Soc Lond Biol Sci. 2012;367(1589):717–730. doi: 10.1098/rstb.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Dreu CK, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328(5984):1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 20.Sheng F, Liu Y, Zhou B, Zhou W, Han S. Oxytocin modulates the racial bias in neural responses to others’ suffering. Biol Psychol. 2013;92(2):380–386. doi: 10.1016/j.biopsycho.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman R, Masalha S, Alony D. Microregulatory patterns of family interactions: Cultural pathways to toddlers’ self-regulation. J Fam Psychol. 2006;20(4):614–623. doi: 10.1037/0893-3200.20.4.614. [DOI] [PubMed] [Google Scholar]
- 23.Hari R, Henriksson L, Malinen S, Parkkonen L. Centrality of social interaction in human brain function. Neuron. 2015;88(1):181–193. doi: 10.1016/j.neuron.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Stanley DA, Adolphs R. Toward a neural basis for social behavior. Neuron. 2013;80(3):816–826. doi: 10.1016/j.neuron.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasson U, Frith CD, Frith CD. Mirroring and beyond : Coupled dynamics as a generalized framework for modelling social interactions. Philos Trans R Soc Lond B Biol Sci. 2016;371(1693):20150366. doi: 10.1098/rstb.2015.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Dev Sci. 2010;13(6):886–899. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- 27.Haegens S, Luther L, Jensen O. Somatosensory anticipatory alpha activity increases to suppress distracting input. J Cogn Neurosci. 2012;24(3):677–685. doi: 10.1162/jocn_a_00164. [DOI] [PubMed] [Google Scholar]
- 28.Kringelbach ML, McIntosh AR, Ritter P, Jirsa VK, Deco G. The Rediscovery of Slowness: Exploring the Timing of Cognition. Trends Cogn Sci. 2015;19(10):616–628. doi: 10.1016/j.tics.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Ki JJ, Kelly SP, Parra LC. Attention strongly modulates reliability of neural responses to naturalistic narrative stimuli. J Neurosci. 2016;36(10):3092–3101. doi: 10.1523/JNEUROSCI.2942-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dmochowski JP, et al. Audience preferences are predicted by temporal reliability of neural processing. Nat Commun. 2014;5:4567. doi: 10.1038/ncomms5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahnakoski JM, et al. Synchronous brain activity across individuals underlies shared psychological perspectives. NeuroImage. 2014;100:316–324. doi: 10.1016/j.neuroimage.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy J, et al. Oxytocin selectively modulates brain response to stimuli probing social synchrony. NeuroImage. 2016;124(Pt A):923–930. doi: 10.1016/j.neuroimage.2015.09.066. [DOI] [PubMed] [Google Scholar]
- 33.Fiske ST. Interpersonal stratification: Status, power, and subordination. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. Wiley; New York: 2010. pp. 941–982. [Google Scholar]
- 34.Tropp LR, Pettigrew TF. Relationships between intergroup contact and prejudice among minority and majority status groups. Psychol Sci. 2005;16(12):951–957. doi: 10.1111/j.1467-9280.2005.01643.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Wu B, Liu Y, Wu X, Han S. Challenging emotional prejudice by changing self-concept: Priming independent self-construal reduces racial in-group bias in neural responses to other’s pain. Soc Cogn Affect Neurosci. 2015;10(9):1195–1201. doi: 10.1093/scan/nsv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: Evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry. 2016;79(3):174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonov V, et al. Brain Development Cooperative Group Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross J, et al. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98(2):694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez R, Griffin D. The correlational analysis of dyad-level data in the distinguishable case. Pers Relatsh. 1999;6:449–469. [Google Scholar]
- 41.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Schneiderman I, Kanat-Maymon Y, Zagoory-Sharon O, Feldman R. Mutual influences between partners’ hormones shape conflict dialog and relationship duration at the initiation of romantic love. Soc Neurosci. 2014;9(4):337–351. doi: 10.1080/17470919.2014.893925. [DOI] [PubMed] [Google Scholar]
- 43.Central Bureau of Statistics 2012 The social poll on religiousness and family 2009–2010 (Central Bureau of Statistics, Jerusalem). Available at www.cbs.gov.il/statistical/seker-chevrati-h123.pdf. Accessed September 5, 2016.
- 44. CBS Jerusalem Israel (2015) Central Bureau of Statistics, Population, by population group report.
- 45.Smooha S. 1992. Arabs and Jews in Israel: Change and Continuity in Mutual Intolerance (Westview Press, Boulder, CO), Vol 2, pp 29–345.
- 46.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage. 2005;24(3):771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Chang WT, et al. Combined MEG and EEG show reliable patterns of electromagnetic brain activity during natural viewing. NeuroImage. 2015;114:49–56. doi: 10.1016/j.neuroimage.2015.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldman R. Mother-Newborn Coding System Manual. Bar-Ilan Univ Press; Tel Aviv: 1998. [Google Scholar]