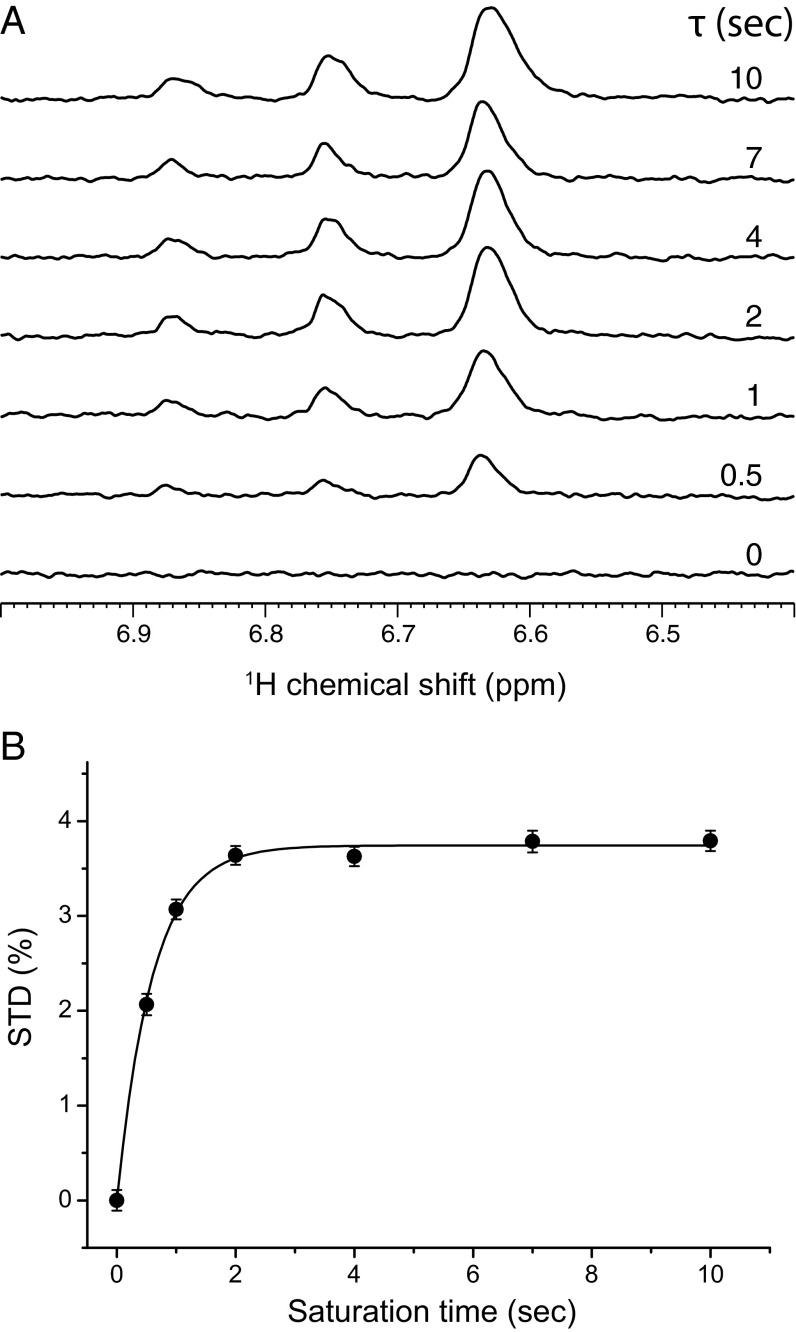
Fig. 1.
1H STD NMR determination of isoflurane interaction with NaChBac. (A) The 1H STD spectra for the region of isoflurane (CF3-CHCl-O-CHF2) resonances at different saturation times as indicated in the figure. The sample contained NaChBac (50 μM) and isoflurane (1.1 mM). Each STD spectrum was obtained by subtracting a pair of spectra acquired in an interleaved fashion with on- and off-resonance frequencies of 0.4 and 25 ppm, respectively (VSTD = Voff – Von). The −CHCl− proton of isoflurane has a single peak centered at 6.63 ppm; the −CHF2 proton has triplet peaks centered at 6.75 ppm with one of the peaks overlapping with the −CHCl− peak. 1H chemical shifts were referenced to the 4,4-dimethyl-4-silapentane-1-sulfonic acid resonance at 0 ppm. (B) STD (%) of isoflurane as a function of the saturation time. STD (%) values were calculated based on (Voff –Von)/Voff × 100%, where Voff and Von were signal integrals of isoflurane in the off- and on-resonance saturation transfer spectra, respectively. Experimental STD (%) values were fit to Eq. 1 as outlined in Methods. Uncertainties in STD (%) were determined by the NMR signal intensity and noise level. At a longer saturation time, STD (%) reached a steady-state regime.