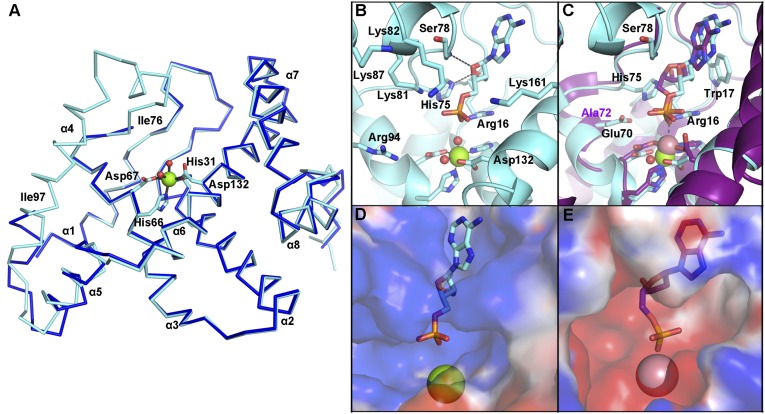
Fig. 2.
OxsA has a mononuclear HD domain phosphohydrolase protein fold that appears designed to bind nucleoside analogs with four-membered rings. (A) The structure of OxsA (blue) is missing residues Ile76–Ile97, which become ordered in the OxsAOXT-P structure (cyan). (B) OXT-P binds within the HD domain active site and interacts with His75 and the backbone of Ser78. Arg16 orients the 5′-phosphate of OXT-P near Mg2+ and within a positively charged pocket. (C) Despite sharing similar architectures, an overlay of E72A-YfbR (purple) with bound dAMP reveals that the 3′-carbon of dAMP would be located unfavorably close (1.4 Å) to the ε-nitrogen of His75. (D) The overall positive electrostatic potential of the OxsA active site. (E) The electrostatic potential measured for the equivalent pocket of E72A-YfbR (14) shows an overall negative charge. In D and E, the electrostatic potential surfaces are displayed on an equivalent scale. Green and pink spheres represent the divalent metal cocrystallized with OxsA (Mg2+) and E72A-YfbR (Co2+).