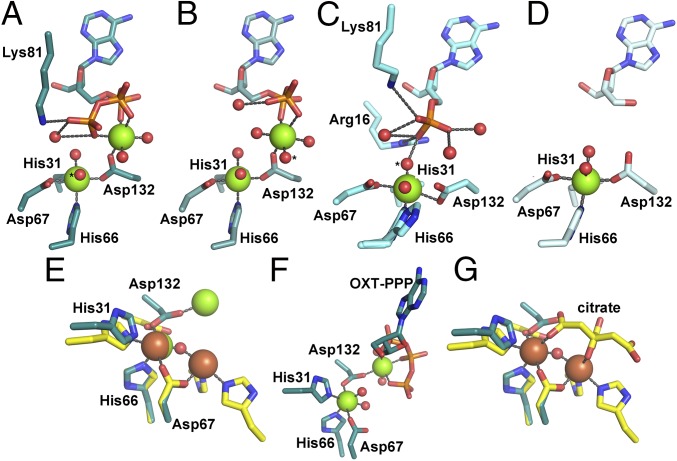
Fig. 4.
The metal content of the OxsA active site is modulated through substrate binding. (A) OxsAOXT-PPP has a dinuclear center with one Mg2+ found in the HD-site and the other ligated primarily by substrate. (B) A similar dinuclear site is observed in OxsAOXT–PP. (C) OxsAOXT–P has a single Mg2+ ligated by the mononuclear HD domain residues. His66 exhibits two conformations. (D) A mononuclear site is also observed in the structure of OxsAOXT. (E) Overlay of OxsAOXT-PPP (dark cyan) with PhnZ (yellow) reveals use of a different Asp as the bridging ligand of the dinuclear site. (F) Mg2+ in the second site in OxsAOXT-PPP is 5.2-Å from Mg2+ in the HD-site and has only one protein ligand. (G) The Fe ions in PhnZ are 3.7 Å apart, bridged by a μ-oxo moiety and a citrate molecule from the crystallization buffer that binds in place of substrate (18). The second Fe site has three protein ligands (18). In A–C, the proposed nucleophilic water is indicated by an asterisk (*). Green, brown, and red spheres correspond to magnesium, iron, and water molecules, respectively. Stereoviews of OxsAOXT-P, OxsAOXT-PP, and OxsAOXT-PPP can be found in Fig. S2.