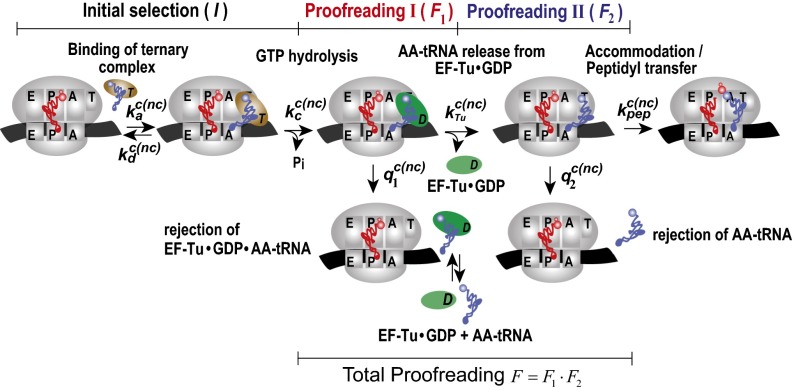
Fig. 1.
Proposed schematic of tRNA selection in bacterial protein synthesis. A ternary complex with aa-tRNA and EF-Tu·GTP binds to A/T state of the pretranslocation ribosome with association rate constant ka. Then, the ternary complex dissociates with rate constant kd or GTP is hydrolyzed with rate constant kc, leading to ribosome-bound ternary complex EF-Tu·GDP·aa-tRNA, which dissociates with rate constant q1, or EF-Tu·GDP dissociates with rate constant kTu, leading to an aa-tRNA−bound preaccommodation state of the ribosome. From this state, aa-tRNA dissociates with rate constant q2 or accommodates into the A site with rate constant kpep. There are three selection steps in this scheme: Near-cognate tRNA can be rejected during initial selection (I), first proofreading step (F1), and second proofreading step (F2). Notations c and nc in rate constants stand for cognate and noncognate reaction, respectively.