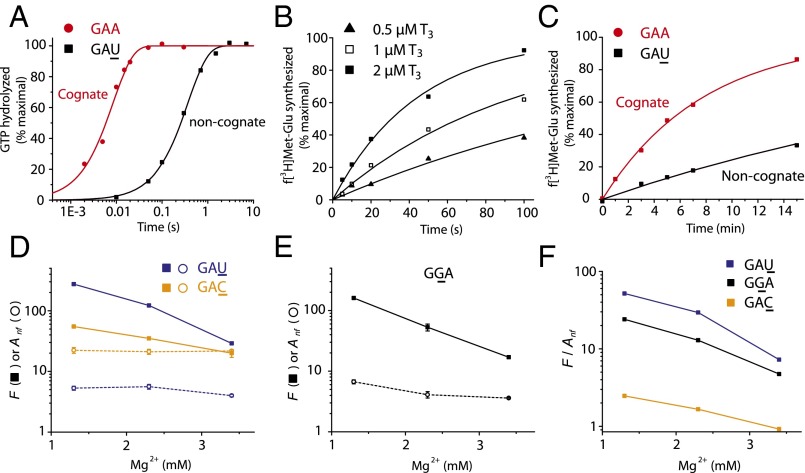
Fig. 2.
The proofreading factor of ternary complex selection converges to the accuracy of tRNA selection without EF-Tu at high Mg2+ condition. (A) Measurements of GTP hydrolysis for native Glu-tRNAGlu ternary complex (T3; 0.5 µM) binding to 70S initiation complex (IC; 2 µM) programmed with a cognate (GAA, curve in red) or near-cognate (GAU, curve in black) codon in the A site. (B) Kinetics of dipeptide formation from Glu-tRNAGlu reading GAU with varying T3 concentration. (C) Kinetics of EF-Tu−free dipeptide formation from Glu-tRNAGlu reading GAA or GAU with 3 µM tRNA (Scheme S1). (D) [Mg2+] dependence of the proofreading factor, F (filled squares), for codon selection by Glu-tRNAGlu in ternary complex with EF-Tu·GTP and of the accuracy (Anf, opened circles) for EF-Tu−free codon selection by Glu-tRNAGlu. (E) Similar to D, Glu-tRNAGlu reading GGA. (F) [Mg2+] dependence of the F/Anf ratio for Glu-tRNAGlu reading cognate GAA versus near-cognate GAU (blue), GGA (black), and GAC (yellow). Experiments in A−C were performed in buffer containing 2.3 mM free Mg2+. Kinetic data in A−C are representative of at least two independent experiments and are fitted to a single exponential model (see SI Materials and Methods). Data in D and E represent weighted averages from at least two experiments ± propagated SD.