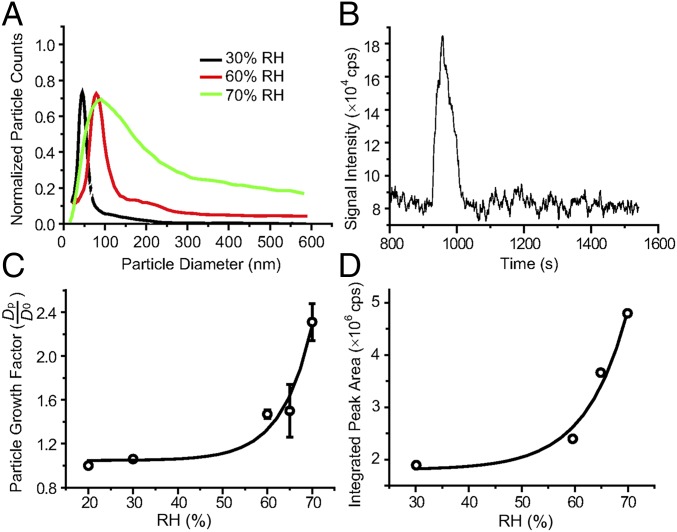
Fig. 3.
Aqueous sulfate formation in the reaction chamber. (A) Evolution in the dry particle size distribution when sized selected oxalic acid particles are exposed to SO2, NO2, and NH3 under three different RH conditions in a 1-m3 reaction chamber. (B) Desorption spectra of particles collected by TD-ID-CIMS after exposure to SO2, NO2, and NH3 at 65% RH. (C) Particle growth factor after exposure to SO2, NO2, and NH3 as a function of RH. The exponential fit is y = 1.05 + 4.0 × 10−5exp (x/6.8) with R2 = 0.96. Each point corresponds to three measurements, and the error bar denotes the SD (1σ). (D) Integrated desorption peak areas of particles collected by TD-ID-CIMS after exposure to SO2, NO2, and NH3 as a function of RH. The exponential fit is y = 1.8 × 106 + 417exp (x/7.9) with R2 = 0.97. All experiments were performed under the dark condition and at temperature of 298 K. The exposure time was 60 min, and the initial particle size was 45 nm. The initial gas concentrations were 250 ppb for SO2 and NO2 and 1 ppm for NH3.