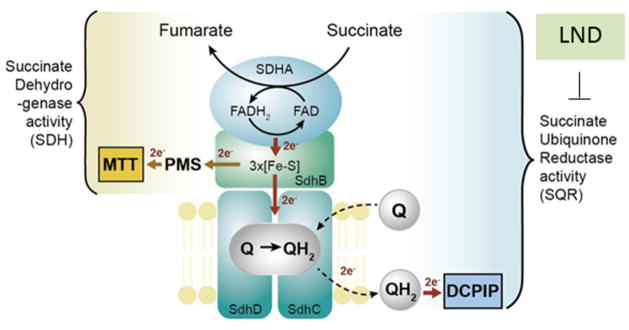
Figure 7. Lonidamine (LND) inhibits the SQR activity of Complex II.
A schematic representation of Complex II subunits and enzyme activities. Complex II contains four subunits: succinate dehydrogenase A (SDHA; flavoprotein), succinate dehydrogenase B (SDHB; iron-sulfur subunit), succinate dehydrogenase C (SDHC; integral membrane protein) and succinate dehydrogenase D (SDHD; cytochrome b small subunit). Flavin adenine dinucleotide (FAD) cofactor bound to SDHA obtains electrons from succinate oxidation. The electrons are then passed to the Fe-S clusters of SDHB and finally to the ubiquinone reduction site within SDHC and SDHD where ubiquinone (Q) is reduced to ubiquinol (QH2). LND inhibits the succinate ubiquinone reductase (SQR) activity of Complex II, whereas it has minimal effect on the SDH of Complex II. SQR activity was measured by electron transfer from succinate to decylubiquinone and 2,6-dichlorophenolindophenol (DCPIP). SDH of Complex II was determined by the electron transfer from succinate to iron-sulfur cluster and finally to phenazine methosulfate (PMS) and 2-(4,5-dimethyl-2-thiazolyl)-3,5-diphenyl-2H-tetrazolium bromide (MTT). Part of this research was originally published in J Biol Chem (52).