Abstract
Purpose
To describe the clinical presentation and management of late (>3.0 years) acute graft rejection in keratolimbal allograft (KLAL) recipients.
Methods
Multicenter, retrospective observational case series. 6 eyes of 6 patients with ocular surface transplant at a mean age of 36.2 years seen at 3 tertiary referral centers for acute graft rejection between 2007 and 2013. Main outcome measures included strength of systemic immunosuppression (SI) at the time of rejection, time to rejection, and clinical presentation of rejection.
Results
Preoperative diagnoses included total limbal stem cell deficiency (LSCD) due to aniridia (n = 2) or chemical injury (n = 4). Following an initially successful outcome, patients experienced late acute graft rejection at a mean time of 67.8 ± 24.1 months (range: 41 to 98) after KLAL while receiving suboptimal levels of SI due to medication taper (n = 5) or noncompliance (n = 1). Objective findings included an epithelial rejection line (n = 6), edema (n = 2), corneal epithelial irregularities (n = 2), and neovascularization (n = 1). Anti-rejection management consisted of topical corticosteroids (n = 6) and augmentation of SI therapy (n = 5).
Conclusion
These cases of late acute graft rejection in KLAL patients support the notion that allodonor cells can persist over the long run and remain at risk for immunologic rejection. It further underscores the fact that long-term success with KLAL may require extension of SI beyond the first few years, albeit at lower levels individualized to each patient.
Keywords: keratolimbal allograft, limbal stem cell transplantation, acute rejection, immunosuppression
Introduction
The maintenance of a healthy corneal epithelium is vital to the optical clarity of the eye. This layer of epithelial cells is constantly undergoing a cycle of regeneration with new cells derived from the multiplication of stem cells located in the basal layer of the limbus.1–3 Severe deficiency of limbal stem cells (LSC), or dysfunction of their local microenvironment, can be a devastating consequence of diverse pathologic insults including congenital aniridia, chemical injury, and Stevens-Johnson syndrome.4–7 Once the function of the LSCs is sufficiently diminished, patients present clinically with various degrees of corneal conjunctivalization, otherwise known as limbal stem cell deficiency (LSCD). Ultimately, the development of significant pain and permanently disabling visual loss are the unfortunate results of non-healing epithelial defects, progressive neovascularization, and severe stromal scarring.
In cases of LSCD refractory to conservative medical therapy, surgical intervention is required. Ocular surface stem cell transplantation (OSST) is used to rehabilitate the ocular surface through restoration of LSCs and the limbal microenvironment. Bilateral LSCD requires allograft transplantation wherein donor stem cells repopulate the corneal epithelium. In particular, keratolimbal allografts (KLAL) utilize cadaver-derived donor limbal tissue and have demonstrated significantly improved visual acuity in patients with bilateral LSCD over several years of follow-up.4, 5
Akin to solid organ transplantation, KLAL recipients may suffer from immune-mediated graft rejection due to the high vascularity and antigen burden of donor limbal tissue.8 Indeed, allograft rejection is the most common cause of long-term KLAL failure. While prior studies have questioned this premise, we have previously reported the presence of donor LSCs up to 3.5 years after KLAL.9, 10 Accordingly, an immunosuppression regimen with multiple systemic agents, in addition to topical drugs, is indicated to prevent allograft rejection.5 However, the appropriate agents, duration, and strength of this treatment schedule are areas of active investigation. In this series, we report six cases of late acute graft rejection (> 3.0 years) after successful KLAL transplantation in patients receiving suboptimal systemic immunosuppression (SI) due to prior regimen tapering protocols or medication noncompliance to further reinforce the need for long-term maintenance therapy in these higher risk patients.
Materials and Methods
A retrospective chart review of patients who underwent KLAL for bilateral LSCD at the Illinois Eye and Ear Infirmary, Cincinnati Eye Institute, and Labbafinejad Medical Center between March 1998 and December 2010 was performed. All patients who suffered from late (> 3.0 years) acute graft rejection between January 2007 and December 2013 after their last successful KLAL operation were included. Patients without adequate documentation of their entire clinical course were excluded. The Institutional Review Board at the University of Illinois at Chicago approved this study. This work was HIPAA-compliant and adhered to the tenets of the Declaration of Helsinki.
Data were collected on patient demographics, LSCD etiology, pre-op and follow-up Snellen best-corrected visual acuity (BCVA), immunosuppression regimens and compliance, ocular complications and interventions during follow-up, characteristics of KLAL failure, treatment of acute graft rejection, adverse events due to SI, and final ocular surface outcome. Clinical characteristics used to identify an episode of acute rejection included pain, decreased vision, or photophobia in addition to one or more of the following: edema and neovascularization of KLAL segments, intense sectoral or 360 degrees of limbal injection, and an epithelial rejection line accompanied by conjunctival injection (Figure 1). Resolution of an acute rejection episode was defined as achievement of a stable ocular surface characterized by an intact corneal epithelium (± residual epithelial irregularities) with absence of inflammatory signs and neovascularization. The KLAL surgical and post-operative immunosuppression protocol varied minimally between the three participating institutions and has been described in prior studies.11–14
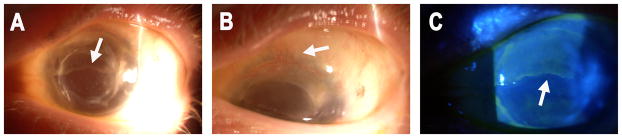
Figure 1.
Clinical characteristics of acute rejection: A, slit-lamp photograph from a 40-year-old woman with aniridia who presented with 6 weeks of decreased vision and increased discomfort and irritation 6 years after KLAL OS while non-compliant with her systemic immunosuppression regimen. Note the epithelial rejection line (white arrow). B, superior corneal neovascularization and conjunctivalization (white arrow). C, use of fluorescein staining to highlight the epithelial rejection line.
Immunosuppression Regimen
Maintenance Therapy
All patients underwent baseline assessment and laboratory investigations 1 month prior to their operation. Topical immunosuppression was initiated immediately after surgery. They received 0.05% difluprednate ophthalmic emulsion QID (1% prednisolone acetate was used before 2008) and were then tapered to a weaker steroid drop to be used indefinitely. In addition, topical cyclosporine (0.05%) was used as adjunctive therapy in patients as they were tapered off systemic agents.
The standard oral immunosuppression protocol was started one week prior to surgery and included prednisone 1 mg/kg QD, tacrolimus (Prograf; Astellas Pharma US, Incorporated, Deerfield, IL) 4 mg BID, and mycophenolate mofetil (MMF; Cellcept; Hoffmann La Roche, Nutley, NJ) 1 g BID. More recently, patients were also concurrently started on valganciclovir (Valcyte; Hoffmann La Roche, Nutley, NJ) 225 mg QD and trimethoprim/sulfamethoxazole (TMP/SMX, single strength; Mutual Pharmaceutical Company, Philadelphia, PA) 1 tablet three times weekly, or dapsone 100 mg QD if the patient has a sulfa allergy, to prevent opportunistic infections while immunosuppressed.
SI was managed both pre- and postoperatively with an organ transplantation team. Standard investigations, including clinical evaluation and various laboratory result monitoring, were performed at 1 month, 3 month, 1 year, and 2 year intervals for the duration of SI therapy.
Prednisone was tapered over 1 to 3 months depending on clinical signs of inflammation. Tacrolimus was titrated to a level of 8 to 10 ng/mL for the first 6 months and 5 to 8 ng/mL afterward for at least 12 to 18 months. Patients with an adequate degree of ocular surface stability were tapered off of tacrolimus and MMF starting at 12 months and 3 years, respectively. However, any history of rejection indicated maintenance of low-dose SI indefinitely if tolerated. Valganciclovir was stopped at 6 or 12 months if the patient is cytomegalovirus IgG positive or negative, respectively. TMP/SMX was discontinued after 1 year.
Each patient’s SI regimen was tailored based upon immunologic risk stratification. Levels of human leukocyte antigen (HLA) matching, panel reactive antibody, donor-specific antibodies, and high-risk status (e.g. young age, severe LSCD or conjunctival disease, repeat OSST likely) determined induction therapy and timing of postoperative tapering.
Acute Rejection Therapy
All acute rejection patients, irrespective of severity, were treated aggressively by augmenting both topical and oral immunosuppression.4 Treatment consists of frequent topical steroids (e.g. 0.05% difluprednate ophthalmic emulsion hourly), subconjunctival injection of triamcinolone, high-dose oral prednisone with tapering over several weeks, and an increase in the dose of concomitant oral immunosuppressive agents.
Results
Eight cases met inclusion criteria; however, only 6 cases, 1 female and 5 male patients, with adequate follow-up data were identified and included in this report (Table 1). Indications for KLAL included total LSCD due to aniridia (n = 2) and chemical injury (n = 4). Ocular comorbidities included keratoconjunctivitis sicca (n = 6) and glaucoma (n = 3). Most patients had undergone prior transplantation including PK (n = 2), KLAL (n = 1), and amniotic membrane transplant (n =1). The mean age at the time of the most recent KLAL surgery was 36.2 years (range: 21 to 52). All patients were started on ≥ 2 SI agents immediately after surgery. Most patients (n = 5) underwent subsequent PK for visual rehabilitation. The mean follow-up time was 110.6 ± 38.4 months and ranged from 80 to 164 months.
Table 1.
Summary of 6 KLAL Patients with Late Acute Graft Rejection
| Case No. | Eye | Age* (yrs) | Sex | Diagnosis | Follow-up Period Interventions | Time to Rejection (months) | SLE Findings | SI at Time of Rejection** | Medical Treatment of Rejection** | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OS | 43 | M | Chemical injury | BCL, OHTN drops | 59.3 | Inferior NV, ERL | MMF 250 mg BID | DFP 0.05% gtt Q1H, MMF 750 mg BID | Partial failure |
| 2 | OS | 26 | M | Anirida | CE + IOL with tube | 98.4 | Superior ERL | Azathioprine 100 mg, Prednisone 15 mg | DFP 0.05% gtt Q1H, Tacrolimus 3 mg BID, MMF 1000 mg BID, Prednisone 7 mg | Partial failure |
| 3 | OD | 52 | M | Chemical injury | Repeat PK x2 | 94.3 | 360° ERL, edema | None | Prednisolone acetate gtt Q1H | Total failure |
| 4 | OS | 22 | M | Chemical injury | OHTN drops | 49.0 | Superior CZN, ERL, CEI | MMF 500 mg, Tacrolimus 1 mg | Prednisolone 1 mg/kg, Betamethasone (high frequency) | Resolved with residual CEI |
| 5 | OS | 38 | M | Chemical injury | Tarsorrhaphy | 70.0 | Superior ERL, edema, CEI, CED | MMF 500 mg Tacrolimus 1 mg | Prednisolone 1 mg/kg, Betamethasone (high frequency) | Resolved with residual CEI |
| 6 | OD | 37 | F | Aniridia | OHTN drops, Diode CPC | 67.0 | ERL | None | Prednisolone acetate gtt Q2H, MMF 500 mg BID | Partial failure |
KLAL = Kerato-limbal allograft; SLE = slit lamp examination; SI = systemic immunosuppression; BCL = bandage contact lens; OHTN = ocular hypertension; NV = neovascularization; ERL = epithelial rejection line; MMF = Mycophenolate Mofetil; DFP = difluprednate; PK = penetrating keratoplasty; CZN = conjunctivalization; CEI = corneal epithelial irregularity; CED = corneal epithelial defect; CPC = cyclophotocoagulation
At time of KLAL procedure.
All medications were oral (PO) and daily (QD) unless otherwise specified.
During the pre-rejection follow-up period, most patients (n = 4) experienced a sustained increase in intraocular pressure controlled with topical medication (n = 2), diode cyclophotocoagulation (n = 1), or a tube procedure (n = 1).
The mean time to acute KLAL graft rejection was 67.8 ± 24.1 months (range: 41 to 98). At the time of rejection, all patients were either on a tapered SI regimen in accordance with prior protocols (n = 5) or noncompliant with their regimen (n = 1). Subjectively, all patients presented with either reduced vision or pain among other complaints including photophobia. Slit lamp biomicroscopy demonstrated an epithelial rejection line in all patients with centripetal progression in most cases (n = 3). Additional features included local or diffuse edema (n = 2), corneal epithelial irregularities (n = 2), neovascularization (n = 1), and conjunctivalization (n = 1).
Medical management consisted of frequent topical corticosteroids in all cases with addition of oral steroids (n = 3) and/or augmentation of other SI agents (n = 3). After aggressive anti-rejection treatment, 2 cases resolved with minor residual epithelial irregularities. However, some patients (n = 3) ultimately developed sectoral ocular surface failure and underwent a repeat ocular surface stem cell transplantation procedure (n = 2). Additionally, 1 patient suffered total ocular surface failure and received a keratoprosthesis device.
At the end of the follow-up period, 5 eyes had a stable ocular surface with (n = 3) or without (n = 2) partial conjunctivalization. The average BCVA before KLAL was −2.2 ± 1.1 logarithm of the minimum angle of resolution (logMAR; ranged from −0.3 to −3.0). The average BCVA at the last follow-up was −0.9 ± 0.3 logMAR (ranged from −0.5 to −1.4). The clinical courses of two representative cases are discussed below.
Case 1
A 43-year-old man with a history of acid burn OS, cataract extraction with intraocular lens implantation and PK presented with a BCVA of hand motion at 2 feet and underwent KLAL. He was immediately started on prednisolone acetate drops QID, moxifloxacin drops QID, a tapering dose of oral prednisone, tacrolimus 3 mg PO BID, and MMF 1000 mg PO BID. He had a repeat PK 3 months after surgery and the prednisone and tacrolimus were tapered and eventually discontinued at 6 months and 1 year, respectively. His interim course was complicated by a persistent epithelial defect and ocular hypertension requiring a bandage contact lens and topical antihypertensive medication. Approximately 59 months after KLAL, while on topical prednisolone acetate QID, MMF 250 mg PO BID, and topical moxifloxacin QID, he presented with pain and photophobia. Slit lamp examination revealed an epithelial rejection line inferiorly with neovascularization and the diagnosis of acute graft rejection was made. He was started on difluprednate 0.05% Q1H and MMF was up-titrated to 750 mg and eventually 1000 mg BID after 2 weeks. Despite mild improvement in ocular surface stability, he went on to develop partial ocular surface failure and underwent combined living related conjunctival limbal allograft and KLAL 3 months later. After this procedure, his course was complicated by multiple episodes of acute PK rejection requiring two repeat PKs at 6 and 7 years after KLAL. At last follow-up, the patient had a stable ocular surface with a BCVA of 20/70.
Case 6
A 37-year-old woman with a history of aniridic keratopathy, progressive LSCD, and glaucoma s/p cataract extraction with intraocular lens implantation presented with a BCVA of counting fingers at 10 feet and underwent bilateral KLAL separated by 10 months (Figure 2). She was immediately started on topical prednisolone acetate QID, prednisone 1 mg/kg PO QD, tacrolimus 4 mg PO BID, and MMF 1000 mg PO BID. Her SI regimen was tapered and discontinued over the course of 3 years. Additionally, her interim course was complicated by elevated intraocular pressure refractory to medication requiring diode cyclophotocoagulation. Five and a half years after her KLAL, she self-discontinued her topical prednisolone acetate TID and presented with pain, redness, and reduced vision in her right eye one month later. Slit lamp examination demonstrated an epithelial rejection line, confirming the diagnosis of acute KLAL graft rejection. Despite augmentation of topical corticosteroids (prednisolone acetate gtt Q2H) and initiation of MMF 500 mg BID, she went on to develop sectoral LSCD in the superior cornea with conjunctivalization extending to the visual axis. At last follow-up, she had a stable ocular surface with sectoral conjunctivalization (150°) and a BCVA of 20/400. The patient has declined any further intervention including repeat sectoral KLAL. The left eye, which did not experience rejection, remains stable at 90 months after KLAL with a BCVA of 20/100.
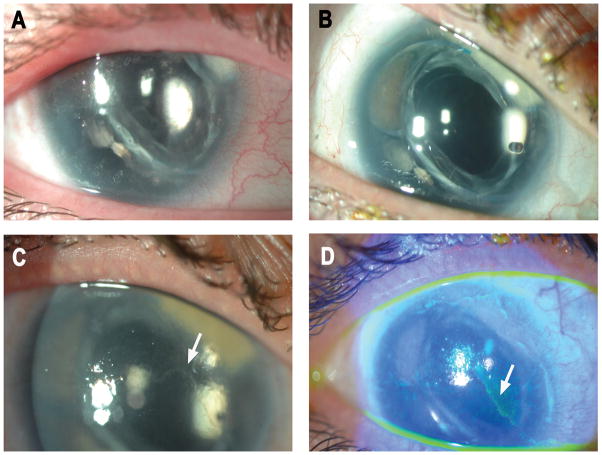
Figure 2.
Case 6: A, preoperative slit-lamp photograph from a 37-year-old woman with aniridia demonstrating epithelial irregularity. B, a stable ocular surface at 2.5 years after keratolimbal allograft while on tacrolimus and mycophenolate mofetil. C, acute graft rejection 5.5 years after surgery evidenced by epithelial rejection line (white arrow). The patient had not received systemic immunosuppression for 2 years and had self-discontinued topical steroids one month prior to presentation. D, use of fluorescein staining to further highlight these findings.
Discussion
In the setting of bilateral total LSCD, KLAL has been widely studied and proven to be an effective form of ocular surface stem cell transplantation.4, 5, 7, 15–17 Significant improvements in corneal epithelial health and visual acuity have been reported in approximately 70% of patients.4, 5, 15 However, KLAL failure is not uncommon and is typically related to graft rejection, persistent inflammation, severe dry eyes and/or adnexal pathology. Indeed, an important challenge with the KLAL procedure is the continued threat of immune rejection, which can lead to progressive loss of graft function over the long-term.4, 12
In contrast to avascular corneal transplants that have relative immune privilege, limbal tissue is highly vascularized and hence the donor cells are readily accessible to the immune system. Graft rejection after KLAL has been well documented in the literature. Reported classification schemes are based upon clinical presentation and include categories such as acute, or severe, and chronic, or low-grade.4, 8 Chronic rejection is more common and, unlike acute cases, may occur with relatively few or no subjective symptoms or objective signs. As a result, it is often difficult to distinguish chronic graft rejection from background inflammation on clinical grounds.
Accordingly, we elected to limit our series to verifiable cases of late-onset acute graft rejection. Prior studies have reported an overall rejection incidence ranging from 13.1% to 46.3% with inadequate immunosuppression frequently identified as statistically significant risk factor.4, 5, 15–19 In the largest study to date, the incidence of rejection was 31.1% over a mean follow-up of 62.7 months.4 Interestingly, the strongest risk factor for rejection was younger age at OSST with the rejection group being more than 10 years younger than the non-rejection group. In fact, there was no significant difference in rejection rates according to diagnosis, inflammatory or otherwise, when adjusted for age. Of particular relevance, noncompliance with immunosuppression also conferred an increased risk of rejection.
In our series, we report the largest number of cases of late-onset acute graft rejection in KLAL patients to date. All patients were found to be insufficiently immunosuppressed due to either down-titration of systemic treatment or regimen noncompliance. The overall mean time to acute rejection was 67.8 months compared with prior studies ranging from 16.9 months for acute rejection in KLAL patients to 19.3 months for severe or low-grade rejection in OSST patients.4,18 In fact, we found that acute rejection could occur as late as 98.4 months postoperatively, which is longer than previously reported.4 This result may be explained by the fact that, unlike earlier protocols, our institutions currently utilize a strict postoperative combined SI regimen. Ultimately, these findings further underscore the long-term threat of rejection and the importance of sufficient SI protection.
Despite appropriate anti-rejection treatment, 2/3 of our cases went on to develop some degree of ocular surface failure, which is consistent with previously reported rejection outcomes.4 We recommend repeat sectoral KLAL in patients with partial ocular surface failure. Alternatively, keratoprosthesis implantation should be considered in cases of total ocular surface failure, particularly in patients with endothelial rejection.
The current study is noteworthy because it provides evidence for the long-term survival of the transplanted limbal stem cells. This notion is in contrast to prior work in which investigators failed to detect donor-derived cells by genetic analysis after months to years of follow-up.10, 20–22 Accordingly, it was suggested that these cells do not survive on a long-term basis and any correlation between the clinical efficacy of limbal transplantation and the survival of donor cells on the ocular surface was called into question. However, in most of these reported cases, subjects did not receive any SI or just short term SI and samples were collected after clinical deterioration had occurred.
In contrast, long-term donor cell survival has been reported in cases in which SI was used. Our group has reported DNA fingerprinting-based detection of non-recipient cells up to 3.5 years after transplantation in patients who were either taking or had received oral immunosuppression.9 Shimazaki et al. found evidence of donor cells in 8 out of 10 eyes in patients with a stable ocular surface at least 300 days after KLAL surgery who were on oral steroids and cyclosporine.23 In a similar study, Reinhard et al. found donor cells up to 56 months after penetrating limbokeratoplasty in patients receiving SI.24 Accordingly, we believe that intense care against immunologic rejection is the key to longer survival of donor-derived epithelial cells and, ultimately, improved KLAL survival.
SI therapy after KLAL is best done in collaboration with an organ transplant team. The optimal dosage and duration of immunosuppression should be individualized. In most cases, patients can decrease the strength of their regimen after the first 18 months depending upon ocular surface stability.4 However, our growing experience with long-term follow-up of KLAL patients and these cases of late acute graft rejection suggest insufficient protection from prior immunosuppression protocols with 1 to 2 year schedules. Accordingly, we recommend maintenance on lower doses for up to 5 years, particularly in younger patients who may be more sensitive to alloantigens.25 Patients with inflammatory disorders, such as Stephens-Johnson syndrome or mucous membrane pemphigoid, have a relatively poor prognosis after KLAL and often require indefinite therapy.7 In addition to such patients with underlying immunologic conditions, any history of rejection should also indicate maintenance on a well-tolerated SI regimen on a long-term basis.
Adverse effects of long-term immunosuppressive therapy in this patient population are minimal, though not non-existent. No major adverse events due to SI therapy were reported during the entire follow-up period of our study. However, we previously reported non-fatal adverse effects in 12/16 patients, nine of whom experienced resolution of these effects during their follow-up period.26 In a large retrospective study of 225 eyes from 136 patients, Holland et al reported 3 severe adverse events in 2 patients (1.5%) with no deaths or secondary tumors.13 There were 21 minor adverse events in 19 patients (14.0%), including increased blood pressure, diabetes, and transient elevations in creatinine and transaminitis. In addition to strict adherence to immunosuppressive therapy, appropriate patient selection, control of ocular comorbidities and frequent postoperative monitoring should be employed in order to minimize the risk of adverse effects.7, 17
In recent years, as a result of these experiences, we have developed a stronger preference for using donor tissue from relatives (whenever available), in order to prolong long-term graft viability. Living-related limbal grafts are associated with a lower risk of rejection compared to KLAL given closer immunologic match.4 In addition to a reduction in the incidence of rejection, improved outcomes may also be achieved as a result of increased likelihood of reaching a state of immunologic tolerance by the host.27
In summary, this series of late acute graft rejection in patients after KLAL provides indirect evidence for the persistence of donor cells up to over 8 years after transplantation. It further confirms that while SI may be successfully tapered off after 3 years in some patients, in some cases, particularly younger patients, long-term systemic therapy is necessary for maintaining graft survival. The external validity of our study is limited by its small sample size, minimal diversity in etiologies of LSCD, the high rejection risk profile of all included patients, and the presence of co-morbidities such as concomitant dry eyes and neurotrophic keratopathy. In addition, biological correlation through the use of DNA fingerprinting techniques would have further strengthened our conclusions. Future studies are needed to identify biomarkers (e.g. systemic or local immunologic markers) that can guide the intensity and duration of SI in these patients.
Acknowledgments
Sources of Funding: Medi Eslani is a recipient of a Clinical Scientist Development Program Award K12EY021475. Ali R. Djalilian is a recipient of an R01 EY024349-01A1, Core grant EY01792 from the NEI/NIH, and an unrestricted grant to the Department of Ophthalmology and Visual Sciences at the University of Illinois at Chicago from RPB.
Footnotes
Meeting Presentation: American Academy of Ophthalmology Annual Meeting, Chicago, 2014.
Conflicts of Interest: Edward J. Holland is a consultant for and receives lecture fees from Allergan; consultant for and receives lecture fees from Abbott Medical Optics; consultant and receives grant support from Alcon Laboratories; consults for Senju Pharmaceutical Co, Ltd; consults for TearScience; consults for TearLab. For the remaining authors none were declared.
References
- 1.Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–1. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 2.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–3. [PubMed] [Google Scholar]
- 3.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–46. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Ang AY, Chan CC, Biber JM, et al. Ocular surface stem cell transplantation rejection: incidence, characteristics, and outcomes. Cornea. 2013;32:229–36. doi: 10.1097/ICO.0b013e318255eac4. [DOI] [PubMed] [Google Scholar]
- 5.Holland EJ, Djalilian AR, Schwartz GS. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003;110:125–30. doi: 10.1016/s0161-6420(02)01451-3. [DOI] [PubMed] [Google Scholar]
- 6.Ontario HQ. Limbal stem cell transplantation: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1–58. [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhtiari P, Djalilian A. Update on limbal stem cell transplantation. Middle East Afr J Ophthalmol. 2010;17:9–14. doi: 10.4103/0974-9233.61211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daya SM, Bell RW, Habib NE, et al. Clinical and pathologic findings in human keratolimbal allograft rejection. Cornea. 2000;19:443–50. doi: 10.1097/00003226-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Djalilian AR, Mahesh SP, Koch CA, et al. Survival of donor epithelial cells after limbal stem cell transplantation. Invest Ophthalmol Vis Sci. 2005;46:803–7. doi: 10.1167/iovs.04-0575. [DOI] [PubMed] [Google Scholar]
- 10.Henderson TR, Coster DJ, Williams KA. The long term outcome of limbal allografts: the search for surviving cells. Br J Ophthalmol. 2001;85:604–9. doi: 10.1136/bjo.85.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croasdale CR, Schwartz GS, Malling JV, et al. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18:52–8. doi: 10.1097/00003226-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Baradaran-Rafii A, Eslani M, Djalillian AR. Complications of keratolimbal allograft surgery. Cornea. 2013;32:561–6. doi: 10.1097/ICO.0b013e31826215eb. [DOI] [PubMed] [Google Scholar]
- 13.Holland EJ, Mogilishetty G, Skeens HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31:655–61. doi: 10.1097/ICO.0b013e31823f8b0c. [DOI] [PubMed] [Google Scholar]
- 14.Nassiri N, Pandya HK, Djalilian AR. Limbal allograft transplantation using fibrin glue. Arch Ophthalmol. 2011;129:218–22. doi: 10.1001/archophthalmol.2010.370. [DOI] [PubMed] [Google Scholar]
- 15.Wylegala E, Dobrowolski D, Tarnawska D, et al. Limbal stem cells transplantation in the reconstruction of the ocular surface: 6 years experience. Eur J Ophthalmol. 2008;18:886–90. doi: 10.1177/112067210801800605. [DOI] [PubMed] [Google Scholar]
- 16.Han ES, Wee WR, Lee JH, et al. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefes Arch Clin Exp Ophthalmol. 2011;249:1697–704. doi: 10.1007/s00417-011-1760-3. [DOI] [PubMed] [Google Scholar]
- 17.Liang L, Sheha H, Tseng SC. Long-term outcomes of keratolimbal allograft for total limbal stem cell deficiency using combined immunosuppressive agents and correction of ocular surface deficits. Arch Ophthalmol. 2009;127:1428–34. doi: 10.1001/archophthalmol.2009.263. [DOI] [PubMed] [Google Scholar]
- 18.Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1278–84. doi: 10.1016/s0161-6420(02)01081-3. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama-Hosoi F, Shimazaki J, Shimmura S, et al. Changes observed in keratolimbal allograft. Cornea. 2006;25:377–82. doi: 10.1097/01.ico.0000176608.65708.27. [DOI] [PubMed] [Google Scholar]
- 20.Swift GJ, Aggarwal RK, Davis GJ, et al. Survival of rabbit limbal stem cell allografts. Transplantation. 1996;62:568–74. doi: 10.1097/00007890-199609150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Williams KA, Brereton HM, Aggarwal R, et al. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am J Ophthalmol. 1995;120:342–50. doi: 10.1016/s0002-9394(14)72164-6. [DOI] [PubMed] [Google Scholar]
- 22.Henderson TR, Findlay I, Matthews PL, et al. Identifying the origin of single corneal cells by DNA fingerprinting: part II-- application to limbal allografting. Cornea. 2001;20:404–7. doi: 10.1097/00003226-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Shimazaki J, Kaido M, Shinozaki N, et al. Evidence of long-term survival of donor-derived cells after limbal allograft transplantation. Invest Ophthalmol Vis Sci. 1999;40:1664–8. [PubMed] [Google Scholar]
- 24.Reinhard T, Spelsberg H, Henke L, et al. Long-term results of allogeneic penetrating limbo-keratoplasty in total limbal stem cell deficiency. Ophthalmology. 2004;111:775–82. doi: 10.1016/j.ophtha.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Joosten SA, Sijpkens YW, van Kooten C, et al. Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int. 2005;68:1–13. doi: 10.1111/j.1523-1755.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- 26.Krakauer M, Welder JD, Pandya HK, et al. Adverse effects of systemic immunosuppression in keratolimbal allograft. J Ophthalmol. 2012;2012:576712. doi: 10.1155/2012/576712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Titiyal JS, Sharma N, Agarwal AK, et al. Live Related versus Cadaveric Limbal Allograft in Limbal Stem Cell Deficiency. Ocul Immunol Inflamm. 2015;23:232–9. doi: 10.3109/09273948.2014.902076. [DOI] [PubMed] [Google Scholar]