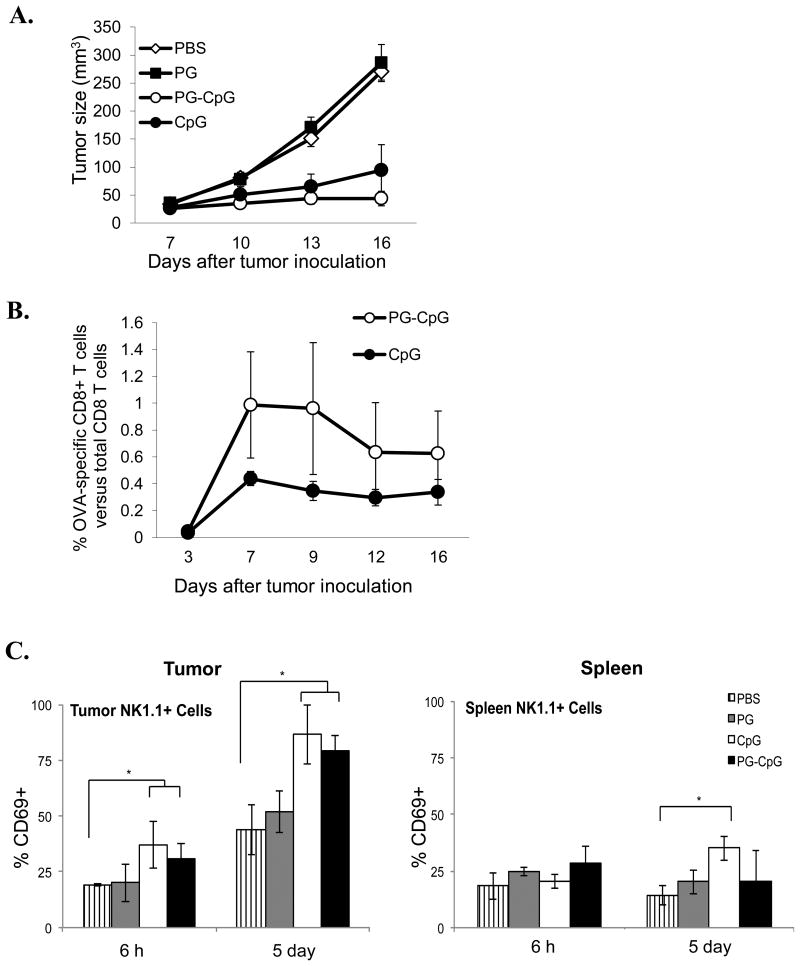
Figure 3. Antitumor efficacy and immunostimulatory potency of PG-CpG in vivo.
Seven days after subcutaneous inoculation of B16-OVA tumor cells in C57BL/6 mice, PG-CpG (50 μg equivalent CpG), CpG (50 μg), or PG (500 μg) was injected into the tumor in 100 μL volume. (A) Tumor growth curves. The mixed-effects linear model was used for the overall comparison of the 4 groups over the entire growth curve. Tumor growth (p<0.0001) was significantly inhibited with PG-CpG or CpG treatment compared to control PBS or PG. (B) Tumor antigen-specific CD8+ T-cell responses. OVA-specific CD8+ T cells from peripheral blood were measured by OVA257-264 peptide-loaded tetramers with flow cytometry. The difference between PG-CpG and CpG was not significant (p = 0.65). (C) NK cell activation. After intratumoral injection of each reagent, cells were collected from tumor (left) and spleen (right) and analyzed for CD69 expression on NK cells. ANOVA was used to assess differences among groups separately by time point (6 h and 5 days) (*p<0.05). Data are from 2 independent experiments with 5 mice/group.