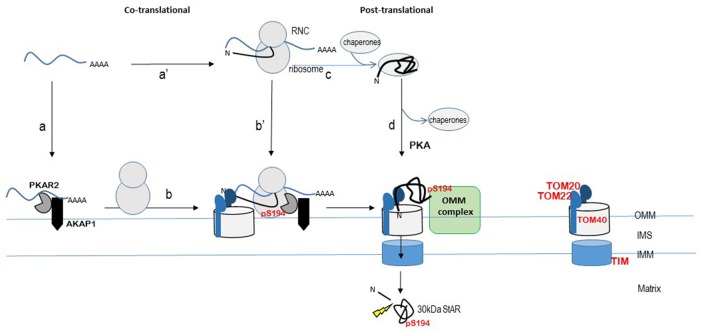
Figure 1.
Model for localized StAR translation and phosphorylation at mitochondria. The TOM20/22-40 complex is an established pathway for import of matrix-localized proteins that contain an N-terminal cleavable signal peptide. StAR is modeled within this import pathway, although the import pathway for StAR remains to be determined. The ribosome nascent chain complex (RNC) is shown as StAR mRNA bound by the ribosome and protein depicted as black line with the N-terminus denoted (N). The START domain is depicted by the “folded” black line. StAR translation is modeled on both free ribosomes (steps c and d) and mitochondria-associated ribosomes (steps a to b or a' to b') as described in the text. Cleavage of the N-terminal signal sequence by matrix metalloproteases (indicated by the thunderbolt) produces the 30 kDa STAR protein (START domain) in the matrix. The import of StAR is the “off switch” for cholesterol transfer due to loss of interaction with an OMM protein complex. The OMM protein complex (indicated here as a green rectangle) that facilitates the StAR-dependent cholesterol transfer from the OMM to the matrix remains to be determined. Proposed OMM components of this complex include StAR, AKAP, PKA, VDAC1/2, TOM22, PCP, TSPO, and ACBD3. The OMM protein that interacts specifically with phosphoStAR to trigger complex formation for cholesterol transfer across the mitochondrial membranes remains to be identified. TIM (Translocase of the inner membrane) complex; IMS, intermembrane space; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane. Concepts of this model have been presented by others in Dyson et al. (2008), Poderoso et al. (2009), Grozdanov and Stocco (2012), Aghazadeh et al. (2015), Prasad et al. (2015), Lee et al. (2016b), Midzak and Papadopoulos (2016), and Paz et al. (2016).