Abstract
Globally, the risk of colorectal cancer (CRC) as well as the incidence of mortality associated with CRC is increasing. Thus, it is imperative that we look at alternative approaches involving intake of non-toxic natural dietary/non-dietary agents, for the prevention of CRC. The ultimate goal of this approach is to reduce the incidence of pre-neoplastic adenomatous polyps and prevent their progression to more advanced forms of CRC, and use these natural agents as a safe intervention strategy during the clinical course of this deadly malignancy. Over the years, pre-clinical studies have shown that silibinin (a flavonolignan isolated from the seeds of milk thistle, Silybum marianum) has strong preventive and therapeutic efficacy against various epithelial cancers, including CRC. The focus of the present review is to provide a comprehensive tabular summary, categorically for an easy accessibility and referencing, pertaining to the efficacy and associated mechanisms of silibinin against CRC growth and progression.
Keywords: colorectal cancer, silibinin, cancer chemoprevention, milk thistle
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States men and women combined; statistical estimates for 2015 indicate ~132,700 new CRC cases and 49,700 associated deaths[1]. Though several initiatives involving colon screening and therapeutic interventions have impacted the overall CRC management in the developed countries of the world[2], clearly more efforts are still needed to overcome CRC risk, to arrest the disease progression, and to reduce the mortality numbers associated with this malignancy. One such effort that has gained an appreciable momentum over the last three decades, involves the intake of non-toxic natural dietary/non-dietary agents which can be used globally, for the prevention of CRC. In most cases, these agents, derived from fruits and vegetables as well as herbs and other supplements, have been shown to reduce the incidence of pre-neoplastic adenomatous polyps and/or their progression to more advanced forms of CRC (Fig. 1)[3-4]. In the present review, an attempt has been made to tabulate and summarize the beneficial effects of one such natural chemopreventive agent i.e., silibinin, against CRC. Silibinin is a flavonolignan which is isolated from the seeds of milk thistle, Silybum marianum (Fig. 2); it has a long history of human use for liver ailments[5], widely available as a nutraceutical supplement and now clinically used to manage hepatotoxicity in various countries including the United States. Importantly, silibinin is considered as a very promising cancer chemopreventive agent based on completed studies showing its strong efficacy against various epithelial cancers (Fig. 3), including CRC[3-4,6-9]. As elaborated in detail in this review, silibinin has shown significant in vitro and in vivo anti-CRC efficacy in various pre-clinical models of CRC. Inferences from completed in depth studies investigating the cellular and biological mechanisms associated with anti-CRC effects of silibinin are summarized in Tables 1–4, which detail the effect of silibinin on various molecules regulating cell cycle, cell survival, autophagy, apoptosis, angiogenesis, and inflammation in its efficacy against colon tumorigenesis.
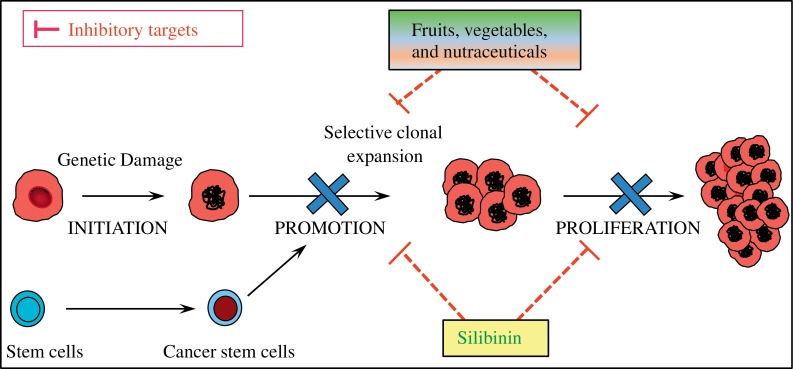
Fig. 1. Chemoprevention by natural dietary or non-toxic nutraceutical agents.
Natural dietary agents, such as silibinin, can reduce the incidence of pre-neoplastic lesions by targeting cancer stem cells and proliferating bulk tumor cells to prevent the disease progression to more advanced forms of the malignancy. Silibinin inhibits tumorigenesis, inflammatory responses, and angiogenesis involved in CRC growth and progression. CRC: colorectal cancer.
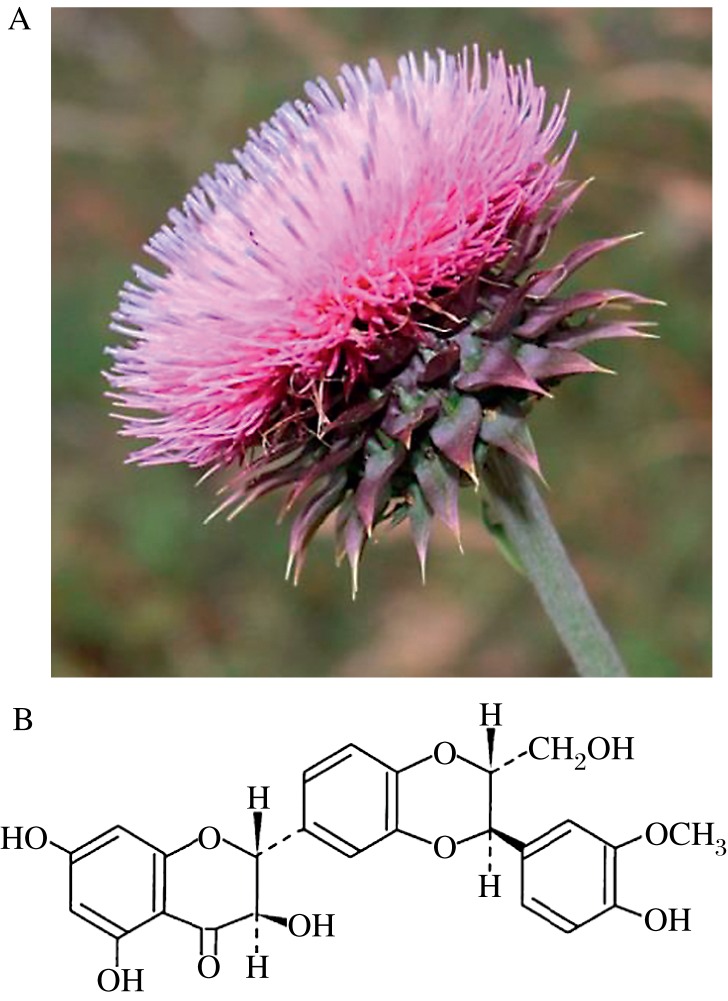
Fig. 2. Milk Thistle.
A: Silybum marianum (Milk Thistle) plant, Family: Asteraceae. B: Chemical structure of silibinin - the principal bioactive constituent of milk thistle extract isolated from the dried seeds of milk thistle.
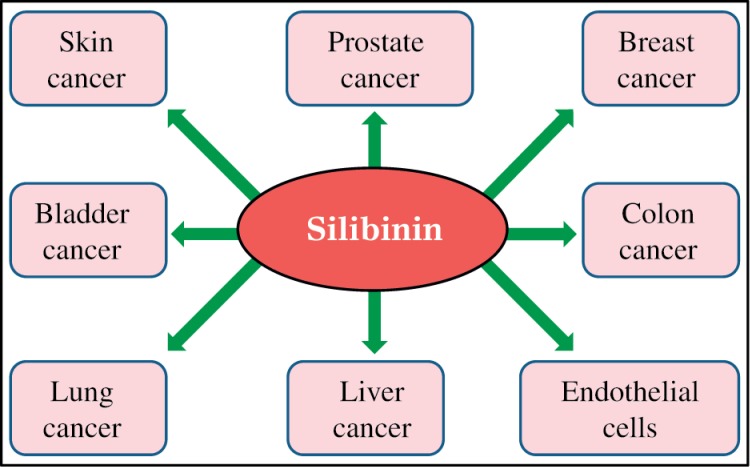
Fig. 3. The targets of silibinin.
Silibinin inhibits various signaling and regulatory pathways in its chemopreventive and therapeutic efficacy against various epithelial cancers.
Table 1. Biological effects of silibinin against human colorectal cancer (CRC) cell lines under in vitro cell culture conditions.
| CRC cell lines | In vitro effects of silibinin | Model and methods | Mechanism of action | Research group |
|---|---|---|---|---|
| HT-29 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fet, Geo, and HCT116 |
|
|
|
|
|
|
|
|
|
| LoVo |
|
|
|
|
|
|
|
|
|
| SW480 |
|
|
|
|
| HT-29, LoVo, and SW480 |
|
|
|
|
| SW480 and SW620 |
|
|
|
|
|
|
|
|
|
| HT-29, LoVo, and SW480 |
|
|
|
|
| SW480 |
|
|
|
|
Abbreviations: CRC, Colorectal cancer; FACS; fluorescence- activated cell sorting; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; RT-PCR, reverse transcription polymerase chain reaction; TCF-4, T-cell factor-4; TRAIL, TNF-related apoptosis-inducing ligand; HDAC, histone deacetylase; DNMT, DNA methyltransferase; SAHA, suberoylanilide hydroxamic acid; IGF-1, insulin-like growth factor-1; EGF, epidermal growth factor; EMSA, electrophoretic mobility shift assay; NMR, nuclear magnetic resonance.
Table 4. Biological effects of silibinin against human colorectal cancer (CRC) tumor xenografts in animal models.
| CRC cell lines | In vivo effects of silibinin | Animal model and treatment modality | Mechanism of action | Research group |
|---|---|---|---|---|
| HT-29 |
|
|
|
|
| LoVo |
|
|
|
|
| SW480 |
|
|
|
|
| SW480 |
|
|
|
|
| Primary human tumor cells and HT-29 |
|
Xenografts of:
|
|
|
| LoVo and SW480 |
|
|
|
|
| SW480 |
|
|
|
|
Abbreviations: CRC, Colorectal cancer; CMC, carboxymethyl cellulose; Rb, retinoblastoma. *silibinin-phytosome (from Indena, Italy) that contains silibinin and phosphatidylcholine in 1:2 ratio.
Silibinin efficacy against CRC cell lines in cell culture
Under in vitro cell culture conditions, silibinin has been shown to inhibit the growth and proliferation of a wide range of CRC cells (Table 1). Mechanistic studies have attributed these inhibitory effects to cell cycle arrest as well as both caspase-dependent and -independent apoptotic pathways (Table 1). An increase in cyclin-dependent kinase (CDK) inhibitors Kip1/p27 and Cip1/p21, together with a decrease in the kinase activity of CDK2 and CDK4 in G0/G1 arrested CRC cells; while an increase in the kinase activity of CDC2/p34 molecule along with a decrease in protein expression of cell cycle regulators cdc25C, cdc2/p34 and cyclin B1 by silibinin in G2M arrested CRC cells have been reported (Table 1). Furthermore, a decrease in the hyperphosphorylation of Retinoblastoma (Rb) by silibinin has also been reported as one of the mechanisms involved in the early cell cycle arrest induced by silibinin in CRC cells; though total Rb levels are unaffected, a decrease in the protein expression levels of cyclin -D1, -D3, -A and -B1 and CDK-1, -2, -4, and -6 by silibinin has been reported. The in vitro effects of silibinin alone or in combination with other molecules are summarized in Table 1[10-17].
Other in depth studies have shown that at physiologically relevant dose (100 µmol/L), silibinin causes oxidative stress in CRC cells, which triggers an apoptotic response, and is followed by a chain of events involving strong inhibition of the PI3K-Akt-mTOR pathway, activation of the ERK1/2 pathway, rough endoplasmic reticulum stress (ER), and suppression of protein translation, which results in autophagy-mediated programmed cell death type II. High-resolution nuclear magnetic resonance spectroscopy (1H, 13C,31P-NMR) studies have further confirmed that silibinin interferes with essential mitochondrial metabolic pathways, and the events associated with both phospholipid as well as protein synthesis[13]. Furthermore, silibinin has also been found to interfere with the glucose uptake in these cells; overall, these events result in energy restriction in CRC cells and their severe and irreparable injury by silibinin[13]. Studies have also shown that significant apoptotic death of CRC cells occurs when high silibinin doses are used[13,18], which suggests that silibinin harbors the potential to induce both apoptotic and autophagy associated programmed cell death in CRC cells.
With regards to silibinin efficacy against CRC stem cells (CSC), silibinin has also been shown to interfere with the kinetics of CSC pool generation, which in turn, decreases the self-renewal and rectifies the aberrant differentiation of the CSC[19-20]. These results are detailed in Table 2; the potential of silibinin to target CSC is being extensively investigated by our group (Table 2)[21-22]. Our in vitro studies showed that silibinin strongly decreases the percentage of colonosphere formation (a stem cell characteristic) of CRC cells and that this effect on CSC is mediated via blocking of interleukin (IL)-4/6 signaling in CRC cell lines. Silibinin also caused a strong decrease in IL-4/6 induced activation of STAT-3 and NF-kB transcriptional activity, which was associated with decreased mRNA/protein levels of various CSC regulatory molecules, and CSC-associated markers and transcription factors (Table 2). We also found that silibinin significantly reduces the booster signals of macrophages towards CSC, resulting in decreased colonosphere numbers under both normoxic and hypoxic conditions (Agarwal & colleagues 2015, unpublished data, Table 2). These results are highly significant, given the fact that silibinin has shown efficacy against both CSC and bulk cancer cells, and that CSC are now primarily recognized as the essential factors contributing to initiation/progression as well as the relapse of CRC[23-26].
Table 2. Biological effects of silibinin against human colorectal cancer stem cells (CSC).
| Colorectal cancer stem cells (CSC) | Effects of silibinin | Model and methods | Mechanism of action | Research group |
|---|---|---|---|---|
| Primary human tumor cells and HT-29 |
in vitro:
|
|
|
|
| HT-29, LoVo, and SW480 |
in vitro:
|
|
|
|
| HT-29 and SW480 |
in vitro:
|
|
|
|
| HT-29 |
in vivo:
|
|
|
|
Abbreviations: CRC, Colorectal cancer; CSC, colorectal cancer stem cells; FACS; fluorescence- activated cell sorting; RT-PCR, reverse transcription polymerase chain reaction; RT2PCR, real -time RT-PCR; EMSA, electrophoretic mobility shift assay; MRI, magnetic resonance imaging; DCE-MRI, dynamic contrast enhanced-MRI; 18FDG-PET, 18Fluorine-2-Deoxy-Glucose (FDG) positron emission tomography.
Notably, several in vitro mechanistic studies have used high doses of silibinin (300 µmol/L); the clinical relevance of such high doses warrants caution; this is founded on the results of several preclinical as well as clinical trial studies that underline limiting the silibinin concentration to ~100 µmol/L dose in in vitro studies based on silibinin concentrations achievable in mouse plasma[27] and colon tissues of CRC patients[28-29]. Specifically, the results of the clinical trial data showed that silibinin feeding in the form of 720 mg/day (formulated with phosphatidylcholine) as ‘silipide capsules’ for 7 days to CRC patients leads to high bioavailability of silibinin in colonic tissue (121 nmol/g of silibinin traced in colonic tissue which is equivalent to 121 µmol/L silibinin concentration).
Efficacy of silibinin against CRC growth and progression in pre-clinical rodent models
Using azoxymethane (AOM) and 1,2-dimethylhydrazine (DMH) as potential carcinogens to induce sporadic CRC in rodent models, various research groups have demonstrated the potential of silibinin to decrease the number of carcinogen-induced aberrant crypt foci (ACF), crypt multiplicity as well as colonic tumors in these colon tumorigenesis animal models (Table 3). To determine the stage specific efficacy of silibinin feeding, different silibinin feeding protocols based on whether silibinin feeding started before the carcinogen exposure (pre-initiation phase), during the carcinogen exposure (initiation phase), after the carcinogen exposure (post-initiation phase), or through the entire period of the study (pre-/-post initiation phase) were carried out; though all silibinin feeding protocols showed efficacy against CRC growth and progression, the percentage of CRC inhibition was stronger in the animals on the pre-/-post-initiation protocol[30-37]. Silibinin has also shown significant efficacy against spontaneous intestinal tumorigenesis in APCmin/+ mice model (Table 3). Both short and long term intervention studies with silibinin have confirmed that total intestinal polyps are significantly reduced in number and size by silibinin feeding[16,38-40]. While a decrease in proliferation index and an increase in apoptotic index were observed in the polyps of silibinin treated mice, the normal crypt-villus regions of the intestine were not affected. Furthermore, our lab has also used a colitis-related AOM/DSS-induced colon tumorigenesis model to assess the role of inflammatory conditions on colon CSC generation and expansion, and their modulation by silibinin[21]. Our results have indicated the protective effect of oral silibinin feeding in this model as evidenced by the absence of large macroadenomas (>2-3 mm) in the colon. This effect was accompanied by minimal colonic inflammation (decrease in recruitment of inflammatory cells); tissue analysis indicated a decrease in the expression of pro-inflammatory cytokines, and associated transcription factors: STAT-3 and NF-kB levels in the colonic tissues (Table 3). A decrease in transformed stem cell population (for CSC pool expansion) was also identified (dual staining for BMI-1 and CD44) in the colonic tissues. Silibinin feeding has also shown significant preventive and/or therapeutic efficacy against human CRC xenograft tumors in athymic nude mice (Table 4)[11-12,27,41]. These studies indicate that not only has silibinin an inhibitory effect on tumor growth, but this inhibitory effect is sustained even after silibinin withdrawal; furthermore, silibinin has also shown significant efficacy against established xenograft tumors[41].
Table 3. Biological effects of silibinin against tumor growth and progression in preclinical animal models of colon tumorigenesis.
| Animal model | In vivo effects of silibinin | Treatment modality | Mechanism of action | Research group |
|---|---|---|---|---|
| Carcinogen-induced sporadic colon tumorigenesis model | ||||
| AOM |
|
|
|
|
| AOM |
|
|
|
|
| AOM |
|
|
|
|
| DMH |
|
|
|
|
| Mouse model of spontaneous intestinal tumorigenesis | ||||
| APCmin/+ mice |
|
|
|
|
| APCmin/+ mice |
|
|
|
|
| APCmin/+ mice |
|
|
|
|
| APCmin/+ mice |
|
|
|
|
| Inflammation based mouse model of colitis associated colon tumorigenesis | ||||
| AOM/DSS |
|
|
|
|
Abbreviations: AOM, azoxymethane; ACF, aberrant crypt foci; DMH, 1, 2- dimethylhydrazine; CMC, carboxymethyl cellulose; DSS, dextran sodium sulphate; CSC, colorectal cancer stem cells * Siliphos or silibinin-phytosome (from Indena, Italy) that contains silibinin and phosphatidylcholine in 1:2 ratio. * silipide (from Indena, Italy) that contains silibinin and phosphatidylcholine in 1:1 ratio.
Conclusion
The summarized studies in four tables provide adequate evidence highlighting the potential of silibinin intake to significantly impact CRC growth and progression. These results also indicate silibinin potential to interfere with CSC pool expansion via targeting both CSC, bulk daughter cells and their inflammatory niche, as well as associated signals involved with the survival and multiplication of colon CSC pool (Fig. 4); the various mechanisms involved in these inhibitory effects are: a) silibinin causes cell cycle arrest resulting in decreased proliferation, and induces multiple programmed cell death mechanisms; b) silibinin interferes with cellular metabolism to induce energy restriction like conditions; and c) silibinin inhibits various signaling and regulatory pathways involved in tumorigenesis, inflammatory responses, and angiogenesis.
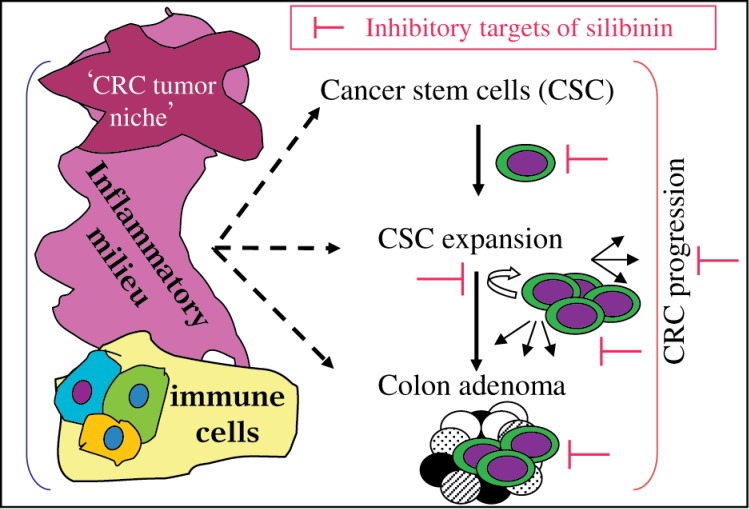
Fig. 4. Targeted proteins of silibinin in CRC.
The inflammatory milieu of the colorectal cancer stem cells (CSC) niche is an important growth regulator for both CSC and progenitor cell population. Silibinin has the potential to target colon CSC self-renewal and aberrant differentiation, and associated inflammatory niche during CRC inhibition.
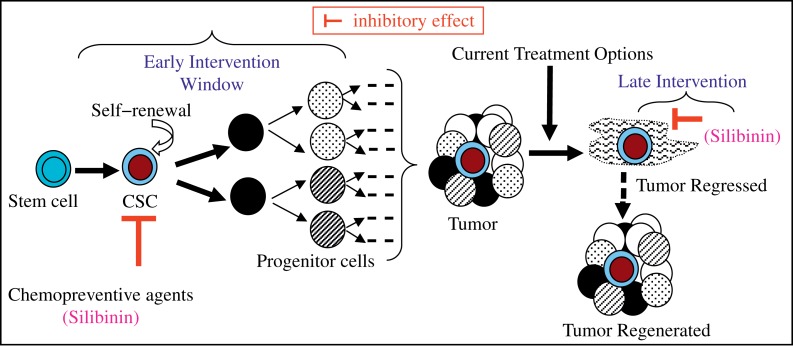
Given that silibinin has the potential to target the CSC or the “tumor initiating cells”, it can be beneficial for use in the early stages of CRC development, as well as in later stages in cancer treatment to eradicate the CSC pool and bulk tumor cells to prevent cancer relapse (Fig. 5). Given the fact that silibinin consumption is exceptionally safe and that it has high bioavailability in colonic tissue of CRC patients, the efficacy evaluation of silibinin in clinical trials is advocated, both as a CRC preventive agent and as an ‘adjunct therapy’ for its clinical use in CRC cases which are incurable by current therapeutic modalities.
Fig. 5. Stem cells or their progenitors transformed into colorectal cancer stem cells (CSC) are considered as major factors responsible for colorectal cancer (CRC).
Novel preventive and therapeutic strategies are needed to reduce CSC number, target their self-renewal capacity or rectify their aberrant differentiation, or interfere with the pro-tumorigenic signals arising in the colon ‘niche’ that affects CSC population. Silibinin acts as a ‘double edged sword'-striking both CRC ‘initiators’ and the ‘initiated’ cells.
Acknowledgment
This work was supported by NCI R01 grant CA112304.
References
- [1].American Cancer Society: Cancer Facts and Figures 2015[J]. Atlanta, Ga: American Cancer Society, 2015. Available online, Last accessed July 1, 2015. [Google Scholar]
- [2].National Cancer Institute : PDQ® Genetics of Colorectal Cancer[J]. Bethesda, MD: National Cancer Institute; Date last modified <07/06/2015>. Available at: http://cancergov/cancertopics/pdq/genetics/colorectal/HealthProfessional. [Google Scholar]
- [3].Raina K, Agarwal R. Promise and potential of silibinin in colorectal cancer management: what patterns can be seen[J]? Future Oncol, 2013, 9(6): 759-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rajamanickam S, Agarwal R. Natural products and colon cancer: current status and future prospects[J]. Drug Dev Res, 2008, 69(7): 460-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Polyak SJ, Morishima C, Lohmann V, et al. Identification of hepatoprotective flavonolignans from silymarin[J]. Proc Natl Acad Sci U S A, 2010, 107(13): 5995-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Raina K, Agarwal R. Combinatorial strategies for cancer eradication by silibinin and cytotoxic agents: efficacy and mechanisms[J]. Acta Pharmacologica Sinica, 2007, 28(9): 1466-1475. [DOI] [PubMed] [Google Scholar]
- [7].Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin[J]. Cancer Lett, 2008, 269(2): 352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ting H, Deep G, Agarwal R. Molecular mechanisms of silibinin-mediated cancer chemoprevention with major emphasis on prostate cancer[J]. AAPS J, 2013, 15(3): 707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Derry MM, Raina K, Agarwal C, et al. Identifying molecular targets of lifestyle modifications in colon cancer prevention[J]. Front Oncol, 2013, 3: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Agarwal C, Singh RP, Dhanalakshmi S, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells[J]. Oncogene, 2003, 22(51): 8271-8282. [DOI] [PubMed] [Google Scholar]
- [11].Kaur M, Velmurugan B, Tyagi A, et al. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling[J]. Neoplasia, 2010, 12(5): 415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kaur M, Velmurugan B, Tyagi A, et al. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft[J]. Mol Cancer Ther, 2009, 8(8): 2366-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raina K, Agarwal C, Wadhwa R, et al. Energy deprivation by silibinin in colorectal cancer cells: A double-edged sword targeting both apoptotic and autophagic machineries[J]. Autophagy, 2013, 9(5): 697-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hogan FS, Krishnegowda NK, Mikhailova M, et al. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer[J]. J Surg Res, 2007, 143(1): 58-65. [DOI] [PubMed] [Google Scholar]
- [15].Akhtar R, Ali M, Mahmood S, et al. Anti-proliferative action of silibinin on human colon adenomatous cancer HT-29 cells[J]. Nutricion hospitalaria, 2014, 29(2): 388-392. [DOI] [PubMed] [Google Scholar]
- [16].Karim BO, Rhee KJ, Liu G, et al. Chemoprevention utility of silibinin and Cdk4 pathway inhibition in APC(-/+) mice[J]. BMC Cancer, 2013, 13: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Woo SM, Min KJ, Kim S, et al. Silibinin induces apoptosis of HT29 colon carcinoma cells through early growth response-1 (EGR-1)-mediated non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) up-regulation[J]. Chem Biol Interact, 2014, 211: 36-43. [DOI] [PubMed] [Google Scholar]
- [18].Kauntz H, Bousserouel S, Gosse F, et al. Silibinin triggers apoptotic signaling pathways and autophagic survival response in human colon adenocarcinoma cells and their derived metastatic cells[J]. Apoptosis, 2011, 16(10): 1042-1053. [DOI] [PubMed] [Google Scholar]
- [19].Kumar S, Raina K, Agarwal C, et al. Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6- mediated survival signals[J]. Oncotarget, 2014, 5(13): 4972-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang JY, Chang CC, Chiang CC, et al. Silibinin suppresses the maintenance of colorectal cancer stem-like cells by inhibiting PP2A/AKT/mTOR pathways[J]. J Cell Biochem, 2012, 113(5): 1733-1743. [DOI] [PubMed] [Google Scholar]
- [21].Tyagi A, Somasagara R, Kumar S, et al. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research[C]. Apr 5-9; San Diego, CA Philadelphia (PA): AACR; Cancer Res 2014, 74(19 Suppl). [Google Scholar]
- [22].Kumar S, Kumar D, Raina K, et al. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research[C]. Pennsylvania, Philadelphia (PA) 2015 Apr 18-22. [Google Scholar]
- [23].Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells[J]. Proc Natl Acad Sci U S A, 2007, 104(24): 10158-10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ricci-Vitiani L, Fabrizi E, Palio E, et al. Colon cancer stem cells[J]. J Mol Med, 2009, 87(11): 1097-1104. [DOI] [PubMed] [Google Scholar]
- [25].Sanchez-Garcia I, Vicente-Duenas C, Cobaleda C. The theoretical basis of cancer-stem-cell-based therapeutics of cancer: can it be put into practice?[J]. Bioessays, 2007, 29(12): 1269-1280. [DOI] [PubMed] [Google Scholar]
- [26].Ward RJ, Dirks PB. Cancer stem cells: at the headwaters of tumor development[J]. Annu Rev Pathol, 2007, 2: 175-189. [DOI] [PubMed] [Google Scholar]
- [27].Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis[J]. Cancer Res, 2008, 68(6): 2043-2050. [DOI] [PubMed] [Google Scholar]
- [28].Hoh C, Boocock D, Marczylo T, et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences[J]. Clin Cancer Res, 2006, 12(9): 2944-2950. [DOI] [PubMed] [Google Scholar]
- [29].Hoh CS, Boocock DJ, Marczylo TH, et al. Quantitation of silibinin, a putative cancer chemopreventive agent derived from milk thistle (Silybum marianum), in human plasma by high-performance liquid chromatography and identification of possible metabolites[J]. J Agric Food Chem, 2007, 55(7): 2532-2535. [DOI] [PubMed] [Google Scholar]
- [30].Velmurugan B, Singh RP, Tyagi A, et al. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats[J]. Cancer Prev Res (Phila), 2008, 1(5): 376-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ravichandran K, Velmurugan B, Gu M, et al. Inhibitory effect of silibinin against azoxymethane-induced colon tumorigenesis in A/J mice[J]. Clin Cancer Res, 2010, 16(18): 4595-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kauntz H, Bousserouel S, Gosse F, et al. Silibinin, a natural flavonoid, modulates the early expression of chemoprevention biomarkers in a preclinical model of colon carcinogenesis[J]. Int J Oncol, 2012, 41(3): 849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sangeetha N, Aranganathan S, Nalini N. Silibinin ameliorates oxidative stress induced aberrant crypt foci and lipid peroxidation in 1, 2 dimethylhydrazine induced rat colon cancer[J]. Invest New Drugs, 2010, 28(3): 225-233. [DOI] [PubMed] [Google Scholar]
- [34].Sangeetha N, Aranganathan S, Panneerselvam J, et al. Oral supplementation of silibinin prevents colon carcinogenesis in a long term preclinical model[J]. Eur J Pharmacol, 2010, 643(1): 93-100. [DOI] [PubMed] [Google Scholar]
- [35].Sangeetha N, Felix AJ, Nalini N. Silibinin modulates biotransforming microbial enzymes and prevents 1,2-dimethylhydrazine-induced preneoplastic changes in experimental colon cancer[J]. Eur J Cancer Prev, 2009, 18(5): 385-394. [DOI] [PubMed] [Google Scholar]
- [36].Sangeetha N, Viswanathan P, Balasubramanian T, et al. Colon cancer chemopreventive efficacy of silibinin through perturbation of xenobiotic metabolizing enzymes in experimental rats[J]. Eur J Pharmacol, 2012, 674(2-3): 430-438. [DOI] [PubMed] [Google Scholar]
- [37].Sangeetha N, Nalini N. Silibinin modulates caudal-type homeobox transcription factor (CDX2), an intestine specific tumor suppressor to abrogate colon cancer in experimental rats[J]. Hum Exp Toxicol, 2014. [DOI] [PubMed] [Google Scholar]
- [38].Rajamanickam S, Kaur M, Velmurugan B, et al. Silibinin suppresses spontaneous tumorigenesis in APCmin/+ mouse model by modulating beta-catenin pathway[J]. Pharm Res, 2009, 26(12): 2558-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rajamanickam S, Velmurugan B, Kaur M, et al. Chemoprevention of Intestinal Tumorigenesis in APCmin/+ Mice by Silibinin[J]. Cancer Res, 2010, 70(6): 2368-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Verschoyle RD, Greaves P, Patel K, et al. Evaluation of the cancer chemopreventive efficacy of silibinin in genetic mouse models of prostate and intestinal carcinogenesis: Relationship with silibinin levels[J]. Eur J Cancer, 2008, 44(6): 898-906. [DOI] [PubMed] [Google Scholar]
- [41].Velmurugan B, Gangar SC, Kaur M, et al. Silibinin exerts sustained growth suppressive effect against human colon carcinoma SW480 xenograft by targeting multiple signaling molecules[J]. Pharm Res, 2010, 27(10): 2085-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yang SH, Lin JK, Chen WS, et al. Anti-angiogenic effect of silymarin on colon cancer LoVo cell line[J]. J Surg Res, 2003, 113(1): 133-138. [DOI] [PubMed] [Google Scholar]
- [43].Yang SH, Lin JK, Huang CJ, et al. Silibinin inhibits angiogenesis via Flt-1, but not KDR, receptor up- regulation[J]. J Surg Res, 2005, 128(1): 140-146. [DOI] [PubMed] [Google Scholar]
- [44].Lin CM, Chen YH, Ma HP, et al. Silibinin inhibits the invasion of IL-6-stimulated colon cancer cells via selective JNK/AP-1/MMP-2 modulation in vitro[J]. J Agric Food Chem, 2012, 60(51): 12451-12457. [DOI] [PubMed] [Google Scholar]
- [45].Raina K, Agarwal C, Agarwal R. Effect of silibinin in human colorectal cancer cells: Targeting the activation of NF-kappaB signaling[J]. Mol Carcinog, 2013, 52(3): 195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kauntz H, Bousserouel S, Gosse F, et al. The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells[J]. Apoptosis, 2012, 17(8): 797-809. [DOI] [PubMed] [Google Scholar]
- [47].Kauntz H, Bousserouel S, Gosse F, et al. Epigenetic effects of the natural flavonolignan silibinin on colon adenocarcinoma cells and their derived metastatic cells[J]. Oncol Lett, 2013, 5(4): 1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]