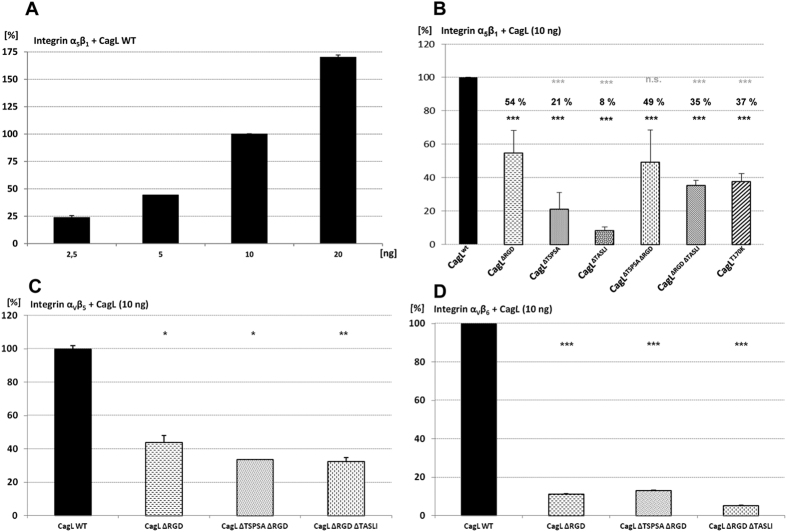
Figure 5. Differential binding of different site-directed CagL motif mutants to recombinant human integrins α5β1, αvβ5 and αvβ6.
Highly purified native CagL motif mutant proteins (ng amounts, as indicated) were incubated in microwell plates coated with recombinant human integrin heterodimers (R&D Systems) for 3 h (followed by extensive washing) and quantitated by an ELISA-like sandwich method using anti-CagL antibody (Methods). The results are depicted as relative values in comparison to the purified CagL wild type control, which was set to 100% (Methods). In (A), the concentration-dependent binding of CagL wild type to integrin is shown; in (B,C,D), the binding of wild type CagL and different CagL mutants (10 ng each) to different integrin variants (α5β1, αvβ5, αvβ6, respectively) is depicted. A significant loss of binding of the mutated CagL variants in comparison to the wild type was quantitated for each integrin type. In panel B, mean values and significances of differences towards CagL wild type binding were calculated by student’s t-test (two-tailed, unpaired) from the mean and SD of three independently performed experiments, summarizing seven data points in total for each protein, and are shown as black asterisks above each bar: ***p < 0.01. Upper row of asterisks in (B) significance of differences between CagLΔRGD mutant and other mutants, respectively, calculated on the basis of the same experiments. In panels (C) and (D), mean and p values from technical duplicates of one experiment, respectively, are depicted. n.s. = not significant.