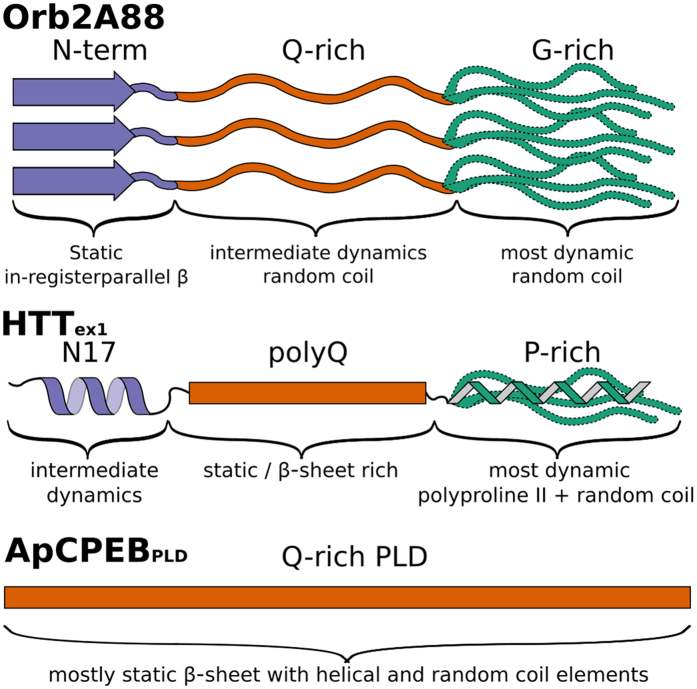
Figure 7. Schematic of the structure and dynamics of Orb2A88 fibrils and comparison with HTTex1 and the prion-like domain of ApCPEB.
In Orb2A88, the N-terminal residues preceding the Q-rich domain are the most static part of the fibril and are in an in-register parallel β-sheet conformation (purple arrow). The Q-rich domain (orange) is disordered and less static than the N-terminus. The G/S-rich domain (green) is highly dynamic and in a random coil conformation. The amyloid core of HTTex1, in contrast, is formed by the polyQ domain, whereas the N-terminus and C-terminus are not part of the amyloid core but form a helix with intermediate dynamics and a dynamic mixed polyproline II, random coil conformation, respectively38,39,49. The relatively large Q-rich, prion-like domain (PLD) of ApCPEB is predominantly in a β-sheet conformation and forms the amyloid core of this protein.