Abstract
Conbercept is a recombinant fusion protein with high affinity for all vascular endothelial growth factor isoforms and placental growth factor. The repeated intravitreal injection of conbercept may cause intraocular pressure (IOP) fluctuations and long-term suppression of neurotrophic cytokines, which could lead to retinal nerve fiber layer (RNFL) damage. This retrospective fellow-eye controlled study included 98 eyes of 49 patients. The changes in IOP and RNFL thickness as well as the correlation between RNFL changes and associated factors were evaluated. The IOP value between the baseline and the last follow-up visit in the injection group and the IOP value of the last follow-up visit between the injection and non-injection groups were not significantly different (p = 0.452 and 0.476, respectively). The global average thickness of the RNFL (μm) in the injection group decreased from 108.9 to 106.1; however, the change was not statistically significant (p = 0.118). No significant difference in the average RNFL thickness was observed at the last follow-up visit between the injection and non-injection groups (p = 0.821). The type of disease was the only factor associated with RNFL thickness changes. In conclusion, repeated intravitreal injections with 0.05 mL conbercept revealed an excellent safety profile for RNFL thickness, although short-term IOP changes were observed.
Anti-vascular endothelial growth factor (VEGF) agents have been widely used for ocular vascular disorders. Recently, intravitreal injection of anti-VEGF agents has become the standard therapy for the treatment of patients with wet age-related macular degeneration (w-AMD)1 and is commonly used for the treatment of diabetic macular edema (DME)2. Therefore, the long-term safety of repeated anti-VEGF injections on the retinal nerve fiber layer (RNFL) has drawn attention. According to the latest meta-analysis, no association was observed between anti-VEGF injections and RNFL thickness changes3. However, those pooled studies mainly focused on ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA, USA) and bevacizumab (Avastin; Genentech, Inc.)4,5,6, which are monoclonal antibodies against VEGF-A7. Information about RNFL changes after other anti-VEGF injections is limited.
Conbercept (KH902; Chengdu Kanghong Biotech Co., Ltd., Sichuan, China) is a recombinant fusion protein designed as a receptor decoy with high affinity for all VEGF isoforms and placental growth factor (PlGF)8. Its efficacy following intravitreal injection has been proven in vivo9,10,11. A phase II, randomized, double-masked clinical trial has compared two dosing regimens including monthly injection (Q1M) and 3 consecutive monthly injection plus as-needed PRN treatment (3 + PRN), and it suggested that either treatment regimen was similarly efficacious9. Most ophthalmologists in China use the 3 + PRN regimen to treat patients with AMD11. The half-life of conbercept has not been calculated in human eyes, but in rabbit eyes is demonstrated to be 4.2 days, which is close to that of bevacizumab (4.3–6.61 days) and longer than that previously reported for ranibizumab (2.88–2.89 days)12. Because conbercept antagonizes two types of neurotrophic cytokines and because it has a higher binding affinity to VEGF and a longer half-life in the vitreous humor12, this agent might cause more RNFL damage than a VEGF-A inhibitor alone. Moreover, intraocular pressure (IOP) elevations immediately after intravitreal injection are known to occur13. Repeated injections may cause IOP fluctuations13 and lead to RNFL damage. In this study, we evaluated RNFL and IOP changes in patients receiving repeated conbercept injections and investigated the correlation between RNFL thickness changes and the associated factors.
Results
Characteristics of the Patients
Ninety-eight eyes of 49 patients (38 patients with w-AMD and 11 patients with DME) were enrolled in this study. Thirty-two (65.3%) patients were male, and 17 (34.7%) patients were female, with a mean age of 66 ± 9 (38–83) years. Only one eye of each patient received intravitreal conbercept injections, and the fellow eyes were included as the control group (non-injection group). The clinical characteristics of all the patients are listed in Table 1. All patients completed the 12 months follow-up period. No significant differences were observed in the baseline RNFL thickness and the IOP between the injection and non-injection groups. No serious complications such as endophthalmitis, sustained IOP elevation or marked anterior chamber reactions were noted during the follow-up period.
Table 1. Baseline characteristics of included patients who received more than three intravitreal conbercept injections for AMD and DME.
| Total | AMD | DME | |
|---|---|---|---|
| Patient (eyes) | 49 (98) | 38 (72) | 11 (22) |
| Injection/Non-injection (eyes) | 49/49 | 38/38 | 11/11 |
| Mean age | 66 ± 9 | 69 ± 7 | 57 ± 9 |
| Sex (male:female) | 32:17 | 25:13 | 7:4 |
| Injection/Non-injection (p) | Injection/Non-injection (p) | Injection/Non-injection (p) | |
| Baseline IOP (mmHg) | 15.3 ± 2.8/15.9 ± 2.9 (0.301) | 15.2 ± 2.9/15.8 ± 3.0 (0.357) | 15.6 ± 2.5/16.1 ± 2.9 (0.658) |
| Baseline RNFL thickness (μm) | 108.9 ± 24.0/109.9 ± 31.4 (0.863) | 101.9 ± 13.8/101.3 ± 11.7 (0.823) | 133.0 ± 35.0/139.6 ± 54.6 (0.738) |
AMD: age-related macular degeneration.
DME: diabetic macular edema.
IOP: intraocular pressure.
RNFL: retinal nerve fiber layer.
IOP Changes
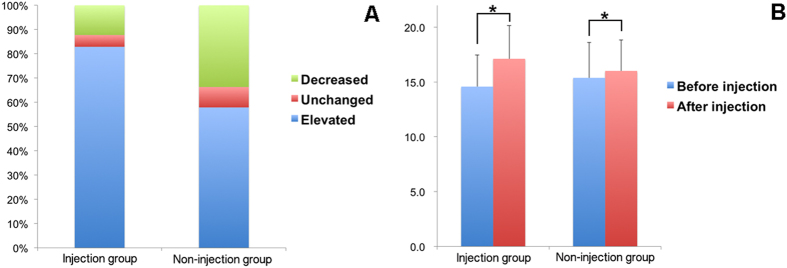
A total of 392 injections were performed in 49 eyes. The IOP change after every injection in both groups was illustrated in Fig. 1. In the injection eyes, IOP elevation was noted after 83% of the injections, and the average IOP elevation after each injection was 2.54 mmHg. In the fellow eyes, IOP elevation was recorded after 58% of the injections, and the average IOP elevation after each injection was 0.64 mmHg. The IOP was significantly elevated after treatment in the injection group (p < 0.01). Additionally, the percentage of IOP elevation in the injection group was significantly higher than that in the non-injection group (p < 0.001).
Figure 1. IOP change 30 min after each injection in the injection and non-injection groups.
(A) In the injection group, the percentages of subjects who experienced an elevation in IOP, no change in IOP, or a decrease in IOP were 83%, 5 and 12%, respectively. The results were 58%, 8 and 34%, respectively, in the non-injection group. (B) The average IOP elevation was 2.54 mmHg in the injection group and 0.64 mmHg in the non-injection group. Both elevations were statistically significant; bars represent standard deviation (*p < 0.01).
In the injection group, the average IOP value of each month’s measurement and the IOP value of the last follow-up visit were 15.5 ± 2.9 and 15.6 ± 2.6 mmHg, respectively. In the non-injection group, the average IOP value of each month’s measurement and the IOP value of the last follow-up visit were 15.5 ± 3.0 and 16.0 ± 2.8 mmHg, respectively (Table 2). The IOP change between the baseline visit and the last follow-up visit in the injection group and the IOP value of the last follow-up visit between the injection and non-injection groups were not significantly different (p = 0.452 and 0.476, respectively). IOP changes from baseline through Month 12 in the injection group and non-injection group were illustrated in Fig. 2. IOP was stable overtime in both groups, and there was no significant difference between the two groups for the mean IOP values throughout the follow-up.
Table 2. Outcomes of included patients who received more than three intravitreal conbercept injections for AMD and DME.
| Total | AMD | DME | |
|---|---|---|---|
| Total number of injections | 392 | 314 | 78 |
| Injection/Non-injection (p) | Injection/Non-injection (p) | Injection/Non-injection (p) | |
| Average IOP (mmHg) | 15.5 ± 2.9/15.5 ± 3.0 (0.887) | 15.5 ± 2.9/15.4 ± 3.0 (0.641) | 15.7 ± 2.8/15.9 ± 2.8 (0.473) |
| Final follow-up IOP | 15.6 ± 2.6/16.0 ± 2.8 (0.476) | 15.7 ± 2.7/16.2 ± 2.9 (0.450) | 15.4 ± 2.5/15.5 ± 2.6 (0.947) |
| IOP spike number | 31/0 | 27/0 | 4/0 |
| IOP elevation during spike (mmHg) | 7.1 ± 2.1 (6.0–16.6) | 7.0 ± 2.1 (6.0–16.6) | 7.7 ± 1.3 (6.3–9.4) |
| RNFL thickness (μm) | |||
| Month 6 | 108.1 ± 26.1/108.3 ± 24.2 (0.971) | 100.9 ± 12.1/102.1 ± 11.5 (0.656) | 135.4 ± 43.8/131.7 ± 41.9 (0.849) |
| Final follow-up | 106.1 ± 19.4/107.0 ± 17.9 (0.821) | 100.6 ± 12.1/102.2 ± 11.9 (0.556) | 125.3 ± 27.4/123.5 ± 25.0 (0.872) |
AMD: age-related macular degeneration.
DME: diabetic macular edema.
IOP: intraocular pressure.
RNFL: retinal nerve fiber layer.
Figure 2. Changes in IOP from baseline through Month 12 in the injection and non-injection group.
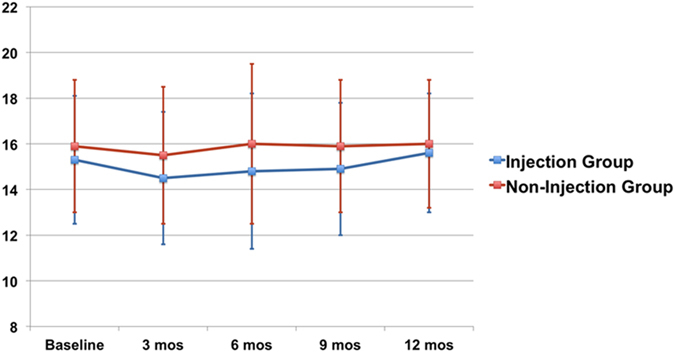
IOP was maintained stable overtime in both groups. There was no significant difference between the two groups for the mean IOP values throughout the follow-up; bars represent standard deviation.
A spike in the IOP (IOP elevation ≥6 mmHg) was observed 31 times (7.9%) 30 min after injection in the injection group, and no spikes were observed in the non-injection group. The average IOP elevation of all spikes was 7.1 ± 2.1 mmHg (Table 2). Among these spikes, only one caused an IOP higher than 25 mmHg at 30 min after the injection, and the IOP returned to normal without any treatment at 60 min after injection. No sustained IOP elevation was detected during the follow-up period.
Changes in RNFL Thickness
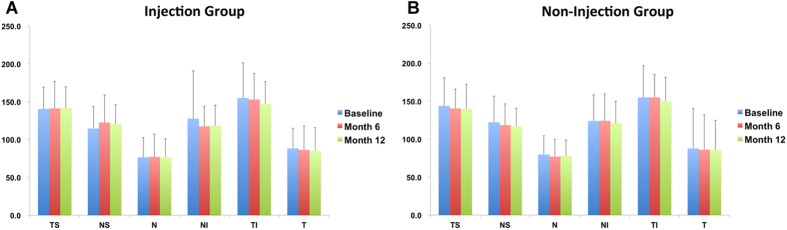
The global average thickness of the RNFL in the injection group decreased from 108.9 ± 24.0 μm at baseline to 108.1 ± 26.1 μm at Month 6 (p = 0.741), and to 106.1 ± 19.4 μm at the last follow-up visit (p = 0.118). The global average thickness of the RNFL in the non-injection group decreased from 109.9 ± 31.4 μm at baseline to 108.3 ± 24.2 μm at Month 6 (p = 0.430), and to 107.0 ± 17.9 μm at the last follow-up visit (p = 0.255) (Table 2). However, the changes were not statistically significant. No significant difference in the average RNFL thickness was observed at the last follow-up visit between the injection and non-injection groups (p = 0.821). Regarding the sector assessment (temporal superior (TS), nasal superior (NS), nasal (N), nasal inferior (NI), temporal inferior (TI), and temporal (T)), no significant change in RNFL thickness in any sector in either group was observed comparing baseline to Month 6 and Month 12 (Fig. 3).
Figure 3. Sector assessment of RNFL thickness changes in the injection and non-injection groups.
(A) In the injection group, no significant change was observed in RNFL thickness in any sector comparing baseline to Month 6 and Month 12. (B) In the non-injection group, no significant change in RNFL thickness in any sector was observed comparing baseline to Month 6 and Month 12; bars represent standard deviation.
Correlation Between RNFL Thickness Changes and Associated Factors
A significant decrease in RNFL thickness (>5 μm)14 was observed in 22.4% (11/49) of patients, accounting for 13.2% (5/38) of the AMD patients and 54.5% (6/11) of the DME patients. The number of IOP spikes, number of injections, type of disease, including AMD and DME, and patient age were included in the multiple logistic regression analysis. Considering the type of disease, patients with DME were more likely to have a decrease in RNFL thickness (p = 0.027) than patients with AMD. The other factors, including injection times, IOP spikes and patient age, were not associated with the change in RNFL thickness (Table 3).
Table 3. Logistic regression analysis of factors associated with RNFL thickness.
| Risk factors | IOP spike* | Injection number | Disease† | Patient age |
|---|---|---|---|---|
| OR | 1.080 | 0.998 | 10.291 | 1.020 |
| 95% CI | 0.446–2.615 | 0.739–1.348 | 1.309–80.884 | 0.924–1.125 |
| p value | 0.865 | 0.989 | 0.027 | 0.695 |
OR: odds ratio.
CI: confidential interval.
*IOP spike was defined as IOP elevation ≥6 mmHg after injection.
†Type of disease, including AMD and DME.
Discussion
Conbercept is a VEGF and PIGF dual antagonist and has become an increasingly common intervention for the treatment of retinal diseases in China. Thus, the safety of this novel drug is of great importance. To the best of our knowledge, this is the first study to investigate the RNFL thickness and IOP changes after repeated conbercept injections.
VEGF and PIGF were once regarded as specific angiogenic factors and currently are implicated in neuroprotection15,16. Theoretically, repeated conbercept injections may lead to IOP fluctuations and chronic suppression of neurotrophic cytokines, which can result in RNFL damage17,18. However, no significant change in RNFL thickness was observed comparing the initial visit to the Month 6 visit and the last follow-up visit in our study. Our result was consistent with previous studies focusing on anti-VEGF-A agents5,6. The decrease in RNFL thickness with increasing age is approximately 0.26 μm per year; therefore, the effect of a natural decrease in RNFL thickness during the follow-up period was negligible19. The reductions in RNFL thickness in AMD and DME patients in our study were 1.3 and 7.3 μm, respectively. The reduction in RNFL thickness in DME patients was significantly higher than that in AMD patients. Moreover, the logistic regression showed that patients with DME were more likely to have RNFL damage. Earlier studies have reported that inner retinal ischemia can cause ganglion cell death and axonal degeneration, resulting in an RNFL defect20,21,22. The RNFL loss in DME patients may be more closely related to vascular abnormalities and retinal ischemia5 than the anti-VEGF and anti-PIGF effects.
The mean IOP values were stable throughout the follow-up in both groups. However, at 30 min after injections, the mean IOP remained significantly above pre-injection values (p < 0.01). IOP spikes were observed 31 times (7.9%) in the injection group, but an IOP value higher than 25 mmHg after 30 min was only recorded once (0.3%). No patients required anti-glaucoma medication. Our results were consistent with previous reports on bevacizumab and Pegaptanib23,24. The general consensus is that a spike in IOP after intravitreal injection of anti-VEGF agents is common and is caused by the increased volume. In most cases, the spike is transient, and routine prophylactic use of IOP-lowering medications is not necessary5,25. Moreover, our study showed that repeated IOP fluctuations would not cause RNFL damage; however, our study did not include any patients with glaucoma. Future studies should examine whether the IOP spikes will harm the already compromised optic nerve in glaucoma patients. Moreover, we could only investigate the correlation between the change in RNFL thickness and the IOP change in this study, and future studies should analyze the correlation between the change in RNFL thickness and the change in the level of intravitreal neurotrophic cytokines.
The current study has several limitations. First, this study is a retrospective study, and the sample size is relatively small. Readers should consider that prospective studies with large sample sizes are needed to confirm our results. Second, we could not standardize the injection intervals because each patient had a different disease course. However, every patient completed the study through the 12-month follow-up period, and we were able to use the fellow eyes as the control group so that all the baseline characteristics were comparable. Additionally, we provided some direct results about the change in RNFL thickness after repeated intravitreal conbercept injections, which indicated that no significant RNFL loss occurred.
In conclusion, conbercept has an antagonistic effect for both VEGF and PIGF. Still, our study showed that repeated 0.05 mL intravitreal conbercept injections were associated with an excellent safety profile when considering the thickness of the RNFL, even though they could lead to short-term IOP changes.
Methods
Subjects
The study retrospectively analyzed the IOP and RNFL data of 49 patients who underwent more than three intravitreal conbercept injections for AMD or DME in one eye and were followed-up for 12 months between January 2010 and December 2014 at Shanghai General Hospital. The treatment indication for patients with AMD was untreated active subfoveal or juxtafoveal CNV. The treatment indication for patients with DME was untreated clinically significant DME. This study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Shanghai General Hospital. Informed consent was obtained from all subjects. All patients had AMD or diabetic retinopathy in both eyes, but the fellow eyes did not require treatment and were observed as the control group (non-injection group). Intravitreal conbercept (0.5 mg/0.05 mL) injection was performed by one retinal specialist (KL). The exclusion criteria were as follows: (1) eyes with a history of glaucoma or suspected of glaucoma; (2) eyes with previous intravitreal injection of triamcinolone acetate, bevacizumab, ranibizumab or conbercept; (3) eyes with a history of treatment that could potentially affect the RNFL, such as cataract extraction, vitrectomy, and laser photocoagulation; (4) eyes combined with other ocular problems including neuro-ophthalmologic diseases, uveitis and refractive error exceeding ± 6.00 diopters; and (5) the condition of the fellow eyes worsened and required laser photocoagulation and/or anti-VEGF injection. Each patient underwent a thorough ophthalmic examination, including best-corrected visual acuity (BCVA), slit-lamp observation, IOP and spectral domain optical coherence tomography (SD-OCT) measurements and fundus photography before the first injection and at each follow-up visit.
IOP Measurement
The IOP values of the study and fellow eyes were analyzed 30 min before and after each injection and at each monthly visit for 12 months. An IOP spike was defined as an IOP elevation ≥6 mmHg after the injection compared with the IOP before the injection5. If an IOP elevation ≥10 mmHg after injection was noted, the patient was asked to stay another 30 min, and the IOP was measured at 60 min. If the IOP was higher than 30 mmHg at 60 min, a topical IOP-lowering medication treatment was prescribed, and the patient was reassessed after 24 hours.
RNFL Thickness Measurement
An SD-OCT (Heidelberg Engineering, Heidelberg, Germany) examination was performed at baseline and at Month 6 and 12 to evaluate RNFL thickness by a single technician who was blinded to the patient information. The following parameters were used for each RNFL thickness acquisition: resolution mode: high speed; circle diameter: 3.5 mm; size X: 768 pixels (10.8 mm); size Z: 496 pixels (1.9 mm); scaling X: 14.05 μm/pixel; and scaling Z: 3.87 μm/pixel. The RNFL thickness from the inner margin of the internal limiting membrane to the outer margin of the RNFL layer was automatically segmented using SD-OCT software (Spectralis 5.1.3.0; Heidelberg Engineering, Heidelberg, Germany). The peripapillary RNFL thickness measurements of the global (G) average and six segments (TS, NS, N, NI, TI, and T) were analyzed automatically by the device.
Other Outcome Measures
The correlations between the RNFL thickness change and potentially associated factors were analyzed. Considering the sample size, four factors that could most likely affect the RNFL after conbercept injection5 were included as follows: (1) IOP spikes; (2) number of injections; (3) type of disease, including AMD and DME; and (4) patient age. To determine the correlation, patients were divided into a group with a significant decrease in RNFL thickness and a group without a significant decrease in RNFL thickness. Regarding the test-retest variability, a change ≤5 μm in average RNFL thickness from baseline was defined as an acceptable change14.
Statistical Analyses
Data analyses were completed using SPSS (Version 13.0, Chicago, IL, USA). Continuous variables (e.g., RNFL thickness and patient age) were expressed as the means and standard deviations. Categorical variables (e.g., sex) were expressed as numbers and frequencies. A paired t-test and Student’s t-test were used to compare RNFL thickness and the change in IOP within groups and between groups. Pearson chi-square and Mann-Whitney U tests were used for categorical data. For the associated factor analysis, logistic regression was performed to determine the odds ratio (OR) and 95% confidence interval (CI). A two-tailed p-value <0.05 was considered statistically significant.
Additional Information
How to cite this article: Zhang, Z. et al. Changes in Retinal Nerve Fiber Layer Thickness after Multiple Injections of Novel VEGF Decoy Receptor Conbercept for Various Retinal Diseases. Sci. Rep. 6, 38326; doi: 10.1038/srep38326 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 81470638) and the Medical Engineering Grant of Shanghai Jiao Tong University (No. YG2015QN19). The funds were used for analysis and interpretation of the data and for writing the manuscript.
Footnotes
Author Contributions The following authors were involved in the design of the study (X.X., K.L.); collection (Z.Z., X.Y., H.J., K.L.), analysis (Z.Z., Y.Q., Y.Z., K.L.), and interpretation (Z.Z., Y.Q., Y.Z.) of the data; and preparation (Z.Z., X.Y., X.X.), revision (Z.Z., H.J., Y.Q., Y.Z., K.L.), and approval of the manuscript (Z.Z., X.Y., H.J., Y.Q., Y.Z., K.L., X.X.).
References
- Horsley M. B., Mandava N., Maycotte M. A. & Kahook M. Y. Retinal nerve fiber layer thickness in patients receiving chronic anti-vascular endothelial growth factor therapy. Am J Ophthalmol. 150, 558–561.e1 (2010). [DOI] [PubMed] [Google Scholar]
- Wells J. A. et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 123, 1351–1359 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. J., Kim S. N., Chung H., Kim T. E. & Kim H. C. Intravitreal anti-vascular endothelial growth factor therapy and retinal nerve fiber layer loss in eyes with age-related macular degeneration: a meta-analysis. Invest Ophthalmol Vis Sci. 57, 1798–1806 (2016). [DOI] [PubMed] [Google Scholar]
- Sobacı G., Güngör R. & Ozge G. Effects of multiple intravitreal anti-VEGF injections on retinal nerve fiber layer and intraocular pressure: a comparative clinical study. Int J Ophthalmol. 6, 211–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. J., Shin K. C., Chung H. & Kim H. C. Change of retinal nerve fiber layer thickness in various retinal diseases treated with multiple intravitreal antivascular endothelial growth factor. Invest Ophthalmol Vis Sci. 55, 2403–2411 (2014). [DOI] [PubMed] [Google Scholar]
- Demirel S., Batioğlu F., Özmert E. & Erenler F. The effect of multiple injections of ranibizumab on retinal nerve fiber layer thickness in patients with age-related macular degeneration. Curr Eye Res. 40, 87–92 (2015). [DOI] [PubMed] [Google Scholar]
- Schmid M. K. et al. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age-related macular degeneration: a trade-off analysis. Br J Ophthalmol. 99, 141–146 (2015). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. Novel VEGF decoy receptor fusion protein conbercept targeting multiple VEGF isoforms provide remarkable anti-angiogenesis effect in vivo. PLoS One. 8, e70544 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 121, 1740–1747 (2014). [DOI] [PubMed] [Google Scholar]
- Qu J., Cheng Y., Li X., Yu L. & Ke X. Efficacy of intravitreal injection of conbercept in polypoidal choroidal vasculopathy: subgroup analysis of the Aurora study. Retina. 36, 926–937 (2016). [DOI] [PubMed] [Google Scholar]
- Cheng Y., Shi X., Qu J. F., Zhao M. W. & Li X. X. Comparison of the 1-year Outcomes of Conbercept Therapy between Two Different Angiographic Subtypes of Polypoidal Choroidal Vasculopathy. Chin Med J (Engl). 129, 2610–2616 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X. & Sun X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther. 9, 2311–2320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. E., Mantravadi A. V., Hur E. Y. & Covert D. J. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol. 146, 930–934.e1 (2008). [DOI] [PubMed] [Google Scholar]
- Tan B. B., Natividad M., Chua K. C. & Yip L. W. Comparison of retinal nerve fiber layer measurement between 2 spectral domain OCT instruments. J Glaucoma. 21, 266–273 (2012). [DOI] [PubMed] [Google Scholar]
- Foxton R. H. et al. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 182, 1379–1390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T. et al. Placental growth factor-2 gene transfer by electroporation restores diabetic sensory neuropathy in mice. Exp Neurol. 227, 195–202 (2011). [DOI] [PubMed] [Google Scholar]
- Nishijima K. et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 171, 53–67 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y. et al. Protective effects of placental growth factor on retinal neuronal cell damage. J Neurosci Res. 92, 329–337 (2014). [DOI] [PubMed] [Google Scholar]
- Sung K. R. et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 116, 1119–1124 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H. W. et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 51, 3660–3665 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi A. M. et al. Retinal nerve fiber layer thinning associated with diabetic peripheral neuropathy. Diabet Med. 29, e106–111 (2012). [DOI] [PubMed] [Google Scholar]
- Shariati M. A., Park J. H. & Liao Y. J. Optical coherence tomography study of retinal changes in normal aging and after ischemia. Invest Ophthalmol Vis Sci. 56, 2790–2797 (2015). [DOI] [PubMed] [Google Scholar]
- Farhood Q. K. & Twfeeq S. M. Short-term intraocular pressure changes after intravitreal injection of bevacizumab in diabetic retinopathy patients. Clin Ophthalmol. 8, 599–604 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group et al. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology. 113, 992–1001 (2006). [DOI] [PubMed] [Google Scholar]
- Frenkel M. P., Haji S. A. & Frenkel R. E. Effect of prophylactic intraocular pressure-lowering medication on intraocular pres- sure spikes after intravitreal injections. Arch Ophthalmol. 128, 1523–1527 (2010). [DOI] [PubMed] [Google Scholar]