Table 2.
C7-debenzylation conditions of 5,6,7-trisubstituted 3-benzylidene-4-chromanone (18e).
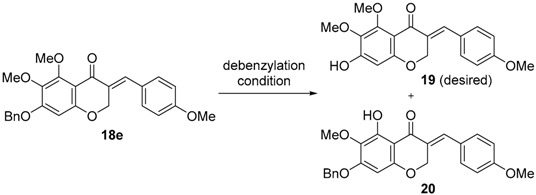
| Entry | Condition | Yield (%) a | |
|---|---|---|---|
| 19 | 20 | ||
| 1 | H2, Pd/C (3 mol %), MeOH, rt | 65 | - |
| 2 | TMSI b, CH2Cl2, rt | - | 60 |
| 3 | HBr (47%), 50 °C | - | 60 |
| 4 | TiCl4 (1 eq), CH2Cl2, 0 °C | NR c | |
| 5 | Li, naphthalene, THF, −25 °C | NR | |
a Isolated yield; b trimethylsilyl iodide; c no reaction.
