Abstract
Purpose:
To evaluate visual outcomes, endothelial cell density and complications following Descemet's membrane endothelial keratoplasty (DMEK).
Methods:
This study included 40 consecutive eyes that underwent DMEK for various pathologies involving the corneal endothelium. Best corrected visual acuity (BCVA) and endothelial cell density (ECD) were measured and compared before and 6 months after surgery.
Results:
Out of 40 eyes, 34 eyes (85%) had BCVA ranging from 0.5 to 0.7 LogMAR 6 months postoperatively. Mean donor ECD was 2367.96 ± 47.87 (range, 2314.0–2472.0) cells/mm2 preoperatively, which was reduced to 1798.42 ± 45.79 (range, 1736.0–1902.0) cells/mm2 6 months after DMEK surgery, indicating a mean reduction of 569.54 cells/mm2 (24%) in ECD.
Conclusion:
DMEK is an emerging and a more advanced alternative to penetrating keratoplasty (PKP) and Descemet's stripping endothelial keratoplasty (DSEK) for corneal pathologies involving the corneal endothelium. Compared to PK and DSEK, however, DMEK has a longer learning curve, and its safety and efficacy need to be confirmed through more experience on a large volume of cases.
Keywords: Descemet's Membrane, Corneal Endothelium, Descemet's Membrane Endothelial Keratoplasty
INTRODUCTION
Corneal endothelial dysfunction and the resulting reduced corneal transparency due to the stromal edema remains a major indication for corneal transplantation. Until 1998, the only known technique for corneal transplantation due to corneal endothelial dysfunction was penetrating keratoplasty (PKP).[1] In 1998, Melles et al published the results of the first successful posterior lamellar keratoplasty. The main advantages of this selective approach are a rapid improvement in visual function, lower incidence of serious postoperative complications, elimination of the suture related complications and providing patient comfort.[1,2] Various techniques of posterior lamellar keratoplasty include Descemet's stripping endothelial keratoplasty (DSEK), Descemet's stripping automated endothelial keratoplasty (DSAEK) and Descemet's membrane endothelial keratoplasty (DMEK). Disadvantages of these techniques are the relatively long learning curve and the high rate of endothelial cell loss (ECL). In the present study, we aimed to evaluate the visual outcome, endothelial cell density (ECD) and complications following DMEK surgery.
METHODS
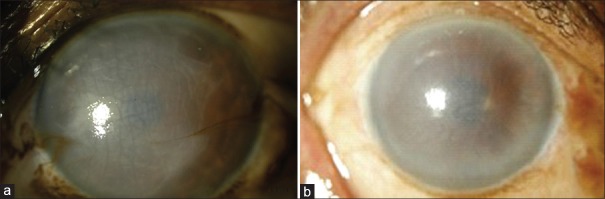
This study comprised of 40 consecutive eyes of 40 patients including 28 male and 12 female subjects with the mean age of 64 ± 10 years. Patients underwent DMEK for endothelial dysfunction due to various pathologies including pseudophakic bullous keratopathy (PBK) [Figure 1a and 1b], Fuchs’ endothelial dystrophy (FED) and failed PKP. An approval from the institutional review board and the ethics committee was taken. All patients signed an informed consent. Corneo-scleral buttons with a death-to-preservation time of <6 hours and ECD of >1800 cells/mm2 were excised and stored in K-SOL (Cornisol, Aurolab, Madurai, India) with the endothelial side up. For donor preparation, Descemet's membrane (DM) was stained with trypan blue (Appasamy Rhex ID-Rx-188, Chennai). The central part of the DM to be transplanted was separated from the posterior stroma using two fine blunt tipped forceps, so that a 7.5 or 8 mm diameter flap of posterior DM with its endothelial monolayer was obtained. Donor preparation was successful in all cases with no tears in DM. Owing to the elastic properties of the membrane, a “Descemet-roll” formed spontaneously, with the endothelium at the outer side. This “Descemet-roll” was then placed in balanced salt solution (BSS).
Figure 1.
(a and b) Images from two different cases with pseudophakic bullous keratopathy (PBK).
A preoperative clinical examination which included best corrected visual acuity (BCVA) using Snellen chart, slit lamp examination, dilated fundus examination using a 90D condensing lens and indirect ophthalmoscopy was performed wherever possible. B scan was done when the fundus details were not clearly visible. All patients were followed-up for 6 months postoperatively. Pre- and postoperative ECD was measured by the eye bank specular and the specular microscope (Topcon Corporation, Tokyo, Japan), respectively. Also, pre-and postoperative findings of BCVA and ECD were compared.
Surgical Technique
In recipient eyes, a 7.5 or 8 mm diameter epithelial mark was made to outline the area of DM removal. A 3 mm tunnel incision was made at the limbus, entering the anterior chamber. An inferior peripheral iridotomy was created and an anterior chamber maintainer was inserted into the anterior chamber. An 8- 9 mm “descemetorhexis” was created using a reverse Sinskey hook (Appasamy associates, AA-1465, Puducherry, India) and DM was stripped from the posterior stroma. The donor Descemet-roll which stained with a 0.08% trypan blue solution (AppasamyRhex ID-Rx-188, Puducherry, India) was sucked into a custom made injector system using an intraocular lens (IOL) cartridge attached to a 1 ml disposable syringe to transfer the tissue from BSS. DM endothelial complex was loaded into the custom made injector. Using the injector system, the donor Descemet-roll was injected into the anterior chamber through the limbal incision. A careful, indirect manipulation of the DM graft was done by tapping over the cornea and slow fluid currents created in the anterior chamber by injecting BSS till the DM roll settled with the edges facing towards the cornea. At this time, the anterior chamber was slightly collapsed by evacuating BSS from the side port incision. Cornea was continuously tapped in the center from the outer epithelial side to unroll the graft with endothelial side down. Simultaneous shallowing of the anterior chamber was done by further draining of BSS from the side port incision. Afterwards, an air bubble was injected underneath the donor DM graft with a 30 G cannula attached to a 1 ml syringe to attach the donor tissue to the recipient posterior corneal stroma. The anterior chamber was completely filled with air for 8 minutes followed by minimal air release in the operating room (OR) to prevent pupillary block [Figure 2]. All patients were examined at postoperative day 1, and months 1, 3, and 6. During each visit, BCVA (expressed in LogMAR) was measured using the Snellen chart. Other examinations included intraocular pressure measurement using a non-contact tonometer and slit lamp examination to reveal any DM detachment [Figure 3a and 3b], and anterior segment optical coherence tomography (Topcon AS-OCT, Topcon Corp, Tokyo, Japan). Postoperatively, all patients were started on a tapering dose of prednisolone acetate eye drops (Pred Forte, Allergan, Indoco, India) initially 6 times a day for 2 weeks and then tapered to 4 times a day for next 45 days and Vigamox (preservative free moxifloxacin eye drops, Alcon Lab Inc.) 4 times a day for one month. After two months, the medications were changed to Dexoren–S eye drops (chloramphenicol 0.5% plus dexamethasone phosphate 0.1%, Indoco Remedies Ltd) 4 times a day for one month and twice a day for next 15 days.
Figure 2.
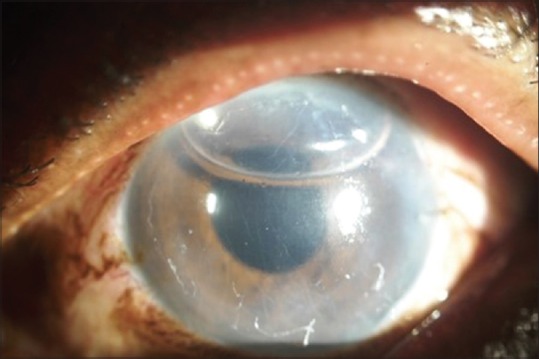
The anterior chamber was completely filled with air for 8 minutes followed by minimal air release in the operating room.
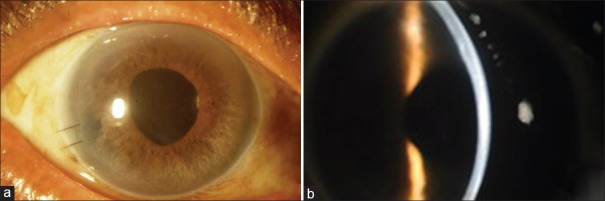
Figure 3.
(a) slit lamp view; (b) slit lamp view with a narrow slit.
Preoperatively, donor ECD and morphology were evaluated in vitro using an inverted light microscope (Eye Bank Kerato Analyzer, EKA-10, Konan Medical, Irvine, CA, USA). The donor endothelium was photographed and evaluated in vivo using a Topcon SP3000p non-contact autofocus specular microscope (Topcon Corp, Tokyo, Japan) at postoperative months 1 and 6. Images of the central corneal window were analyzed and three measurements of ECD were averaged.
Statistical Analysis
The data was collected and tabulated using Microsoft Excel and Word (Microsoft Office 2010, USA). Descriptive and inferential statistical analyses were performed in the present study. Results on continuous measurements were presented as mean ± standard deviation and range and results of categorical measurements were presented as number (percentage, %). Significance was considered at 5% level. A paired t-test (two tailed, dependent) was used to find the significance of study parameters on continuous scale preoperative ECD of the donor and postoperative ECD in the patients.
RESULTS
A total of 40 eyes of 40 patients including 37 cases with PBK, 2 subjects with FED and one patient with failed PKP underwent DMEK. Mean patient age was 64 ± 10 (range, 54 to 74) years. All patients had a pre-existing IOL and there was no concomitant surgery with DMEK. No intraoperative complications were encountered. Mean preoperative uncorrected visual acuity (UCVA) was 1.50 ± 0.24 (range, 1.00–1.80) LogMAR which improved to 0.60 ± 0.60 (range, 0.50–0.70) LogMAR at postoperative month 3 (P < 0.001). Afterwards, postoperative UCVA remained unchanged during longer follow-up [Table 1]. Mean preoperative BCVA was improved from 1.40 ± 0.20 (range, 1.00–1.60) LogMAR to 0.30 ± 0.07 (range, 0.20–0.40) LogMAR three months postoperatively (P < 0.001) and remained stable during further follow-up [Table 2].
Table 1.
UCVA evaluation at pre- and postoperative assessments assessment

Table 2.
BCVA evaluation at pre- and postoperative assessments

Partial DM detachment occurred in 2 eyes (5%) for which air injection was performed. These two eyes had a large peripheral iridotomy. After repeat air injection, patients were kept on strict supine position for 2 hours and then the air was released. There was no graft dislocation or any other complications postoperatively. Slit lamp examinations showed that all corneas remained clear during the 6 months of follow-up. Rejection was not observed in any eye during the follow-up. Mean ECD of the donor tissue was 2368.0 ± 47.87 (range, 2314.0 to 2472.0) cells/mm2 preoperatively that was significantly reduced to 1798.0 ± 45.79 (range, 1736.0 to 1902.0) cells/mm2 6 months after operation. The mean reduction in ECD was 569.0 cells/mm2 (24%, P < 0.001) [Table 3].
Table 3.
Endothelial cell count; evaluation at pre- and postoperative assessment

DISCUSSION
Descemet's membrane endothelial keratoplasty is an emerging and a more advanced alternative to PKP and DSEK for corneal pathologies involving the corneal endothelium. This finding is consistent with the other studies.[3,4] Eighty percent of patients had a BCVA of ≥0.50 to 0.70 LogMAR after 6 months. Mean ECL was 24% after 6 months postoperatively. In 2009, Ham et al[3] reported a case series of 50 patients with FED undergoing DMEK. Of these, over 90% achieved BCVA ≥20/40 (6/12). Mean ECL was approximately 30% in their study. Ten (20%) patients underwent DSEK due to DMEK failure.[3] Similarly, Tourtas et al showed an ECL of 40.97% in a study on 38 eyes that underwent DMEK.[5] Price et al reported 60 eyes undergoing DMEK with an ECL of 32% at 6 months with 94% having a BCVA ≥20/40 (6/12).[6] Guerra et al reported 136 eyes undergoing DMEK with one year follow-up. They showed an ECL of 31% in 3 months and 36% in one year with a BCVA of ≥20/30 (6/9) in 98% of patients.[7] ECL was lower in our study, compared to the other studies.[3,5] The difference can be explained by the method we used for donor DM preparation and attachment. Two blunt corneal forceps were used to strip the donor DM from its posterior stroma and continuously tapped the epithelial side of the cornea in the recipient's eye with partially collapsed anterior chamber for unrolling the donor DM, avoiding direct manipulation of DM graft. The postoperative BCVA in the present study was lower compared to other studies, majority of them included patients with Fuch's endothelial dystrophy showing better results postoperatively. The mean age of patients was also higher in the current study.
In conclusion, our study shows that DMEK is a feasible procedure. This study however has a few limitations such as the small sample size of study population. In order to draw reliable conclusions, similar studies with larger population size are required. Moreover, a longer follow-up period is necessary to comment on the long term efficacy and safety of DMEK. There is no direct comparison between the efficacy of DMEK and other posterior lamellar keratoplasties such as DSAEK in our study. British, Australian, and American people of European ancestry have deeper anterior chambers compared with Chinese, Mongolians and people living in other Asian countries.[8] As the current study was based on Asian people with relatively shallow anterior chamber and difficulty in DM graft manipulation, our results are noteworthy. With all these factors in view and considering visual outcome and ECD as clinical outcome parameters, the incidence and severity of complications, DMEK may soon be preferred over PKP and DSEK/DSAEK for the management of various endothelial pathologies requiring the transplant of only healthy donor endothelium.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Melles GR, Eggink FA, Lander F, Pels E, Rietveld FJ, Beekhuis WH, et al. A surgical technique of posterior lamellar keratoplasty. Cornea. 1998;17:618–626. doi: 10.1097/00003226-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Bahar I, Kaiserman I, McAllum P, Slomovic A, Rootman D. Comparison of Posterior lamellar keratoplasty techniques in penetrating keratoplasty. Ophthalmology. 2008;115:1525–1533. doi: 10.1016/j.ophtha.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Ham L, Dapena I, van Luijk C, van der Wees J, Melles GR. Descemet membrane endothelial keratoplasty (DMEK) for Fuchs endothelial dystrophy: Review of the first 50 consecutive cases. Eye. 2009;23:1990–1998. doi: 10.1038/eye.2008.393. [DOI] [PubMed] [Google Scholar]
- 4.Guerra FP, Anshu A, Price MO, Price FW. Endothelial keratoplasty: Fellow eyes comparison of Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea. 2011;30:1382–1386. doi: 10.1097/ICO.0b013e31821ddd25. [DOI] [PubMed] [Google Scholar]
- 5.Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012;153:1082–1090. doi: 10.1016/j.ajo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Price MO, Giebel AW, Fairchild KM, Price FW., Jr Descemet's membrane endothelial keratoplasty: Prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–2368. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet's membrane endothelial keratoplasty: Prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 2011;118:2368–2373. doi: 10.1016/j.ophtha.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Aung T, Nolan WP, Machin D, Seah SK, Baasanhu J, Khaw PT, et al. Anterior Chamber depth and the Risk of Primary Angle Closure in 2 East Asian Populations. Arch Ophthalmol. 2005;123:527–532. doi: 10.1001/archopht.123.4.527. [DOI] [PubMed] [Google Scholar]