Abstract
The Trier Social Stress Test (TSST) was employed to study response to social evaluative threat in male adolescents with Autism Spectrum Disorder (ASD, n = 21) and typical development (n = 13). Participants wore a mobile electrocardiogram to collect heart rate data. There were significant group effects on respiratory sinus arrhythmia (RSA), a measure of parasympathetic nervous system function, with lower values in ASD (F = 4.97). Bivariate correlations also showed a significant relationship between parent reports of social problems and RSA response to the TSST (r = −0.586). These findings suggest that autonomic dysregulation may contribute to social deficits in adolescents with ASD.
Keywords: Respiratory sinus arrhythmia, Autism spectrum disorder, Adolescence, Social evaluative threat, Psychophysiology
Introduction
The autonomic nervous system (ANS) is a core biological regulatory system; it is thought to underlie a host of approach and avoidance behaviors that drive social interaction (Porges and Furman 2011). Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by a primary deficit in social behavior (APA 2013). Early theoretical models have suggested that a dysregulated ANS may underlie social approach behavior alterations in ASD (Hutt et al. 1964). Because ASD is associated with a wide range of symptoms and co-occurring conditions that impact a number of biological systems (Mazzone et al. 2012), the ANS is a likely candidate for understanding aspects of the biological underpinnings of ASD.
Both the parasympathetic and sympathetic branches of the ANS project to the sino-atrial node of the heart, and are responsible for variations in heart rate. One measure of ANS function is respiratory sinus arrhythmia (RSA), which is a metric of high frequency heart rate variability (HRV) associated with spontaneous breathing, and is thought to measure parasympathetic nervous system (PNS) or ‘rest and digest’ function via cholinergic vagus nerve projections to the heart (Porges 1992). High baseline RSA values are thought to be related to adaptive social functioning, with studies in TD children indicating that high baseline RSA is associated with less social inhibition and greater empathic responsiveness (Diamond et al. 2011). In contrast, RSA decrease was associated with psychosocial stress (Pico-Alfonso et al. 2007) and effortful emotional regulation during exposure to negative or upsetting stimuli (Butler et al. 2006). Overall, in TD populations, high baseline RSA is thought to represent the ability to engage flexibly and adaptively with the social world (Porges 1992), skills that are often challenging for individuals with ASD.
There is an emerging ASD literature regarding ANS effects on cardiac function in pre-pubertal children; although these studies have been somewhat mixed (for review see Benevides and Lane 2015). This may be due in part to differences in the type of stressor or challenge used. For example, studies of school-age children with ASD using cognitive or sensory processing tasks have found reduced RSA. In one such study employing a visual search task, the authors found reduced high frequency HRV decreases in response to challenge in the ASD group compared to TD control children (Daluwatte et al. 2012). Another study of HRV in children with pervasive developmental disorder found reduced parasympathetic responsivity during a visual search task with a high attentional load (Althaus et al. 2004). One study that investigated RSA changes in response to sensory challenge found blunted responsivity in ASD children compared to TD children (Schaaf et al. 2015). Studies that employ facial recognition tasks have been more mixed, with two studies reporting no group differences in RSA (Bal et al. 2010; Watson et al. 2012) and one study reporting reduced RSA responsivity in children with ASD compared to TD children (Vaughan Van Hecke et al. 2009). Taken together, the literature in pre-pubertal children with ASD suggest blunted PNS response, but adolescents with ASD are understudied.
In typical development, the adolescent period is critical for the development of key brain regions subserving social functions, including cortical regions linked to the processing of social information, social judgment, and emotional regulation (Eiland and Romeo 2013) as well as the development and stabilization of neuroendocrine feedback loops that regulate the stress response (McCormick and Mathews 2010). RSA may serve as a downstream marker of regulatory ability related to cortical development or dysfunction (Beauchaine 2015). Evidence from TD populations implicates developmental effects on ANS measures such as RSA that occur during puberty (Tanaka et al. 2000; Kowalewski et al. 2007; Jarrin et al. 2015) and correlate with age, with the greatest effects in the adolescent epoch beginning at the onset of puberty (Shahrestani et al. 2015). RSA studies in TD populations suggest ANS stabilization that occurs between the ages of four and five, and then again during adolescence with the onset of puberty (Pitzalis et al. 2000; Woodall and Matthews 1993), followed by a gradual decline with aging (De Meersman 1993; Jennings and Mack 1984). These studies in TD populations suggest that characterization of a pubertal sample of ASD adolescents is warranted.
Adolescence is a time of increasing social complexity, wherein peers take on more importance and salience relative to adults (Brown et al. 1986; Roisman et al. 2004). Adolescence is also linked to increased stress, in part due to peer bullying and social judgment (Espelage et al. 2001), but also due to the increasing external and environmental demands that are placed on individuals as they begin the transition to adulthood (Compas et al. 1995). This period is also linked to the onset of internalizing problems such as anxiety (Hayward et al. 2003). Anxiety can be operationalized as a state of heightened stress or physiological arousal during anticipation of a real or perceived potential threat; anxiety disorders occur when chronic anxiety interferes with day-to-day functioning (APA 2013). An emerging literature suggests high rates of co-occurring internalizing disorders, including anxiety disorders, throughout the lifespan in ASD (Gjevik et al. 2011; Charlot et al. 2008; Matson and Nebel-Schwalm 2007), as well as increased anxiety symptoms (Muris et al. 1998). For example, many individuals with ASD demonstrate an altered physiological stress response to social stimuli; some children with ASD have a heightened response, and others a blunted response to social stimuli (Corbett et al. 2012). Several studies have demonstrated correlations between RSA and parent- and self-reported anxiety symptoms in ASD (Guy et al. 2014; Kushki et al. 2014), as well as operationalized anxious behaviors in observational studies of attachment in children with ASD (Moskowitz et al. 2013), indicating that co-occurring conditions may play a prominent role in physiologic variability within ASD. The increasing nuance needed to understand and navigate the social world with the onset of adolescence likely creates a significant challenge to ASD adolescents. Surprisingly, little work has been done to investigate the psychophysiological correlates of social stress in the adolescent period, despite the fact that co-occurring problems such as anxiety may have significant impact on functional abilities and quality of life for youth with ASD (Dubin et al. 2015; Pellecchia et al. 2015).
There have been almost no RSA studies that use naturalistic social situations that include real-time social actors. One such study measured RSA during completion of a 10 min play-based observational assessment of social behavior with an adult; in this study, baseline RSA in children with ASD was correlated with frequency of social behavior during the observational assessment of social behavior. Specifically, higher baseline RSA was associated with more conventional gestures and sharing behavior with the adult social actor (Patriquin et al. 2013). However, no studies to date have used peer actors to investigate RSA reactivity in adolescents with ASD.
Measuring the response to social stress is often conducted by examining a known trigger of the stress response, social evaluative threat or social judgment (Mason 1968). One classic experimental paradigm used to study social evaluative threat is the Trier Social Stress Test (TSST), first developed for use in adults (Kirschbaum et al. 1993) and later adapted for use in children (TSST-C, Buske-Kirschbaum et al. 1997). Briefly, participants are given 5 min to prepare a speech in front of two unsupportive adult raters. They then have 5 min to deliver the speech, followed by 5 min of serial subtraction and a debriefing period. In child and adult populations without neurodevelopmental disorders, the TSST has demonstrated reliable increases in the stress response using both neuroendocrine (for review, see Foley and Kirschbaum 2010) and psychophysiological markers of stress responsivity (for review, see Allen et al. 2014). When the TSST has been implemented in neural-cardiac studies of children with ASD, findings have been mixed; two studies have reported reduced responsivity in ASD (Mikita et al. 2015; Hollocks et al. 2014) and one study found no differences (Levine et al. 2012) from TD controls. This inconsistency in findings is likely due to methodological differences in study design, including differences in the type of physiological marker measured, as some studies have focused on group differences in RSA, and others on within-group heart rate changes between baseline and the task. The RSA literature in ASD is likely complicated not only by differences in task design, but also by heterogeneity in ASD samples, both within and across studies. Recent expansion of the diagnostic criteria for ASD has contributed to this heterogeneity, and underscores the importance of a carefully characterized clinical sample (APA 2013). This characterization includes co-occurring psychiatric conditions common in ASD, such as anxiety disorders (van Steensel et al. 2011), which may further complicate and confound investigations of ANS function. The contribution of anxiety symptoms to the stress response in ASD is critical, as some individuals with ASD may be indifferent to social evaluation, whereas others may be hypersensitive to it (Corbett et al. 2012).
The purpose of this study is to examine social stress in adolescents with ASD. In order to measure physiological arousal and regulatory capacity in response to social stress, we measured PNS function using RSA. RSA can be measured in a minimally invasive way, via electrocardiogram, and therefore allows for mobile measurement of ANS effects on cardiac output in realtime. In order to invoke social stress, we employed the TSST, which requires participants to actively engage and interact flexibly with novel social actors in realtime. To date, there have been no studies of pubertal adolescents with ASD that have measured RSA during the TSST. We adapted the TSST for use in a carefully clinically characterized adolescent sample by replacing one of the adult raters with an age-matched peer adolescent to provide more social salience to the task. Previous study has shown enhanced responsivity to peers in ASD, particularly novel peers (Corbett et al. 2010), which is in line with the increased salience of peers during this developmental period. We hypothesized that (1) there would be significantly more within-group variability in reported RSA within the ASD group compared to the TD group (2) adolescents with ASD would show lower RSA at baseline than TD adolescents (3) ASD adolescents would show a decreased stress response to the TSST compared to TD adolescents, and (4) RSA decrease would be correlated with social and anxiety symptoms in ASD, and that, in line with Porges’ Polyvagal Theory (1995) RSA would be associated with ASD symptom severity measures as a metric of degree of social engagement.
Methods
Participants
TD participants were recruited by word-of-mouth, from university-wide email announcements, community events, and fliers. Participants with ASD were recruited via word of mouth and referral from university-based clinical practices, area schools, community advocacy group mailers, community events, and healthcare providers. We recruited and consented all subjects within accordance of ethical standards set forth by Vanderbilt University Institutional Review Board Human Subjects protocols. All procedures performed in this study were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Twenty-five ASD (four female) and 17 TD (four female) adolescents between the ages of 12 and 18 years participated in this study (see Table 1 and Supplemental Table for demographic information). All TD participants had no current or past history of psychiatric or developmental disability or current psychotropic medication usage. ASD participants were not currently taking any antipsychotics or steroid medications and those prescribed stimulants abstained from usage immediately prior to and on the day of the behavioral paradigm, as these substances interfere with the physiologic stress response (Granger et al. 2009). Specifically, eight participants were not taking any medications at the time of the TSST, and five were prescribed a stimulant medication that they abstained from on the day of the TSST. The remaining participants were taking an SSRI (n = 6), melatonin (n = 2), and one participant was also prescribed Baclofen, a GABA receptor agonist prescribed PRN that was not taken on the day of the TSST. Some participants were on more than one medication type, hence the sum of medication subclasses is larger than the total sample n.
Table 1.
Final sample demographics, males only
| Group (N) | Age (SD) | PDS score (SD) | Verbal IQ (SD) | Performance IQ (SD) | FSIQ (SD) | SCQ total score (SD) | SRS total t score (SD) |
|---|---|---|---|---|---|---|---|
| TD (13) | 14.77 (1.70) | 2.80 (0.60) | 118.15 (14.44) | 112.92 (9.33) | 117.69 (11.63) | 2.23 (2.09) | 42.08 (3.12) |
| ASD (21) | 14.99 (1.35) | 3.04 (0.46) | 104.24 (23.90) | 108.86 (15.92) | 107.19 (20.93) | 21.67 (10.48)*** | 75.00 (10.92)*** |
PDS Pubertal Development Scale, FSIQ Full Scale IQ, SCQ social communication questionnaire, SRS Social Responsiveness Scale
p < 0.05;
p < .01;
p < 0.0001
The present study consisted of two lab visits, the first of which involved administration of clinical assessment to confirm diagnosis of ASD and neuropsychological measures. The following forms were completed on the first visit to the laboratory:
Materials
Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler 1999)
The WASI is a short, reliable measure of intelligence and is normed for individuals ages 6–89. It consists of four subtests to assess verbal and nonverbal reasoning abilities. The WASI has strong psychometric properties with high construct validity and internal consistency (Wechsler 1999; Canivez et al. 2009). In ASD populations, the WASI has demonstrated construct validity and predictive accuracy with long form versions of the Wechsler Intelligence Scale (Minshew et al. 2005; Merchan-Naranjo et al. 2011). In this study, the WASI was used as a screening measure; both ASD and TD participants completed the WASI and were required to have a Full-Scale IQ of at least 70.
Autism Diagnostic Observations Schedule Version II (ADOS-II, Lord et al. 2012)
Adolescents with ASD completed the ADOS-II, Schedule 3 or 4, depending on developmental appropriateness, to confirm the presence of ASD. Research reliable clinicians (BAC or CN) administered the ADOS. In the instance of first-time diagnoses, the Autism Diagnostic Interview-Revised (ADI-R, Lord et al. 2012) was also administered to a parent. All ASD participants met ADOS-II criteria for ASD with a total ADOS score greater than 7. Two participants had ADOS-II scores of 6 were includes in the study and were determined to have ASD on the basis of developmental history, SCQ scores, ADI, and clinical judgment (BAC, CN).
The Pubertal Development Scale (PDS)
The PDS (Petersen et al. 1988) is a parent report measure of the degree of physical development on relevant variables including growth in height, growth in body hair, skin changes/acne, and overall development compared to peers. Gender specific factors are also ascertained, including voice deepening and facial hair questions for boys, and breast growth and menarche questions for girls. Scores range from from 1: “change has not yet begun” to 4: “development complete”. All participants were required to have a mean score of at least 2, indicating pubertal onset.
Child Behavior Checklist (CBCL)
The CBCL is a parent report of problem behaviors that occur in childhood across multiple domains and can be used to identify behaviors indicative of psychiatric problems in children ages 6–18 (Achenbach et al. 1991). The questionnaire asks parents to rate the frequency of behaviors using a Likert scale ranging from 0 = “Not True” to 2 = “Very Often True”. Scores are summed to create a t-score for various syndromes. Parental reports of children with ASD indicate on average higher scores across all symptom domains than the general population, with the Attention, Anxiety, Social, and Internalizing Problems subscales the most commonly endorsed (Schroeder et al. 2011). Given our a priori interest in social stress and anxiety in the present study, we examined the Anxiety and Social Problems subscales, as well as the Internalizing Problems subscale, which is a summed measure of the symptoms reported in the following subscales: Anxious/Depressed, Withdrawn/Depressed, and Somatic Complaints.
The Social Communication Questionnaire (SCQ)
The SCQ (Rutter 2003), is a brief parent-report questionnaire that assesses for past and present behaviors indicative of ASD. The SCQ has high sensitivity (.88–.92) and specificity (.62–.72) for diagnosis of ASD (Chandler et al. 2007; Witwer and Lecavalier 2007). In the present study, we used the SCQ as a screening tool to rule out ASD in the TD group and to corroborate ASD diagnosis. SCQ scores greater than or equal to 15 are thought to indicate ASD. In this study, no TD participant had a SCQ score greater than 10.
The Social Responsiveness Scale Second Edition (SRS-2)
The SRS (Constantino and Gruber 2005) is a parent-report questionnaire used to assess the presence of ASD symptoms across the following domains: Social Awareness, Social Motivation, Social Cognition, Social Communication, and Restrictive and Repetitive Behaviors. The SRS identifies the severity of ASD symptoms and also can be used to differentiate social impairments in ASD from those that occur in other diagnoses. It has high sensitivity (.74–.80) and specificity (.69–1.00) for the identification of ASD and is highly correlated with the ADOS, the gold standard of ASD diagnostic measures (Bolte et al. 2011). A total t-score was calculated for each participant and was the primary variable of interest in our analyses of clinical symptom severity. Average scores in a nonclinical sample range from 40 to 60 and, in the present study, no TD participant had a t-score greater than 50.
Procedures
To calculate RSA, participants wore a mobile multichannel BioNex 8-slot chassis electrocardiogram unit that included a 4-channel biopotential amplifier (MindWare Technologies, Gahanna, OH) during the TSST behavioral paradigm visit. Upon arrival for the TSST, familiar lab personnel escorted participants to a testing room. After allowing for 20 min to adjust to the new environment, lab personnel introduced the mobile ECG unit with a cartoon depicting electrode placement on the torso. Participants were then shown the electrodes and allowed to “practice” placement on their hand to allow for sensory accommodation to the sensor adhesive and gel. This practice mediated potential ANS responses to sensory novelty. Lab personnel then placed sensors on the participant’s torso following a standard 5-lead ECG protocol. Participants sat quietly for 5 min for collection of baseline ECG data. The participant wore the mobile unit for the remainder of the paradigm.
Following completion of the baseline ECG, participants began the social stress protocol. The TSST is a psychosocial paradigm known to elicit a physiological stress response in a controlled laboratory setting (Buske-Kirschbaum et al. 1997). The TSST is a 20-min paradigm consisting of four 5-min components: (1) Preparation Period, (2) Speech Delivery Period, (3) Serial Subtraction Period, and (4) Debriefing Period (see Fig. 1).
Fig. 1.
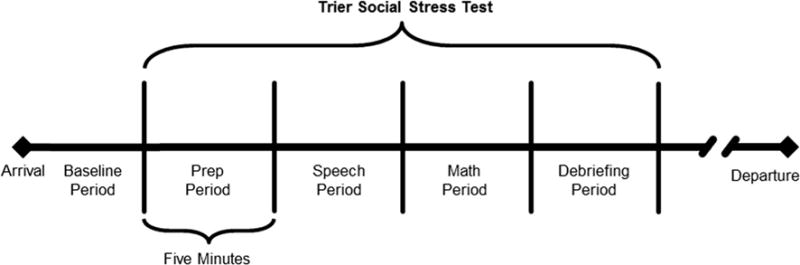
Trier Social Stress Test Paradigm
Adolescents were escorted to the TSST room, which was a conference room where they were greeted by two raters wearing white lab coats and holding clipboards, sitting behind a table. A video camera was set up behind the raters to film the participant during the paradigm. Both trained raters did not to provide verbal or nonverbal feedback during the TSST and each maintained a neutral affect throughout the protocol. In order to increase the salience of social judgment for the adolescent sample, one of the raters was an age-matched peer of the same gender. One rater was female and one rater was male. The adult rater asked the participant to stand at a microphone facing the table, and then informed the participant that they had 5 min to prepare a speech about why they would be the best candidate for a job. They would then be asked to deliver this speech before the selection committee of experts, and that their speech would be compared to others’.
The participant then returned to the testing room and sat quietly for 5 min of preparation. Participants were not permitted to take notes or use aides of any kind, and any questions directed at lab personnel were ignored. At the end of 5 min, the participant returned to the TSST room, where they then had 5 min to deliver their speech. If the participant stopped speaking before the 5 min time period ended, the adult rater prompted them with a series of questions delivered in a neutral tone, such as, “Tell us about your previous job experience” and “Why should we pick you for the job over everyone else?” Following the speech delivery period, raters instructed the participant to begin the serial subtraction task, which requires them to subtract the number 7 from 758 serially out loud. If the participant made a mathematical error, the rater told them to stop and begin again from the beginning. If the participant made more than five errors, the rater asked them to subtract by three, starting at 307. After 5 min, the raters begin debriefing the participant by explaining that their performance will not be judged and that the entire task was “just pretend.” The raters show positive affect, praise the participant’s performance, and provide support and encourage the participant to ask questions. The raters then thank the participant taking part in the study.
We calculated RSA minute by minute from each participant’s ECG trace using the BioLab 2.4 Heart Rate Variability Software Suite provided by MindWare Technologies. This software suite allows for automated recognition of R peaks on the ECG trace. We inspected and processed all data to confirm the presence of a clear R peak and the absence of cardiac abnormalities such as arrhythmias. In the case of R peaks not detected by the automated software, R peaks were marked by hand following visual inspection by a trained rater who identified them on the basis of their characteristic shape (EKE). In the absence of a clear R peak, a rater added the point that was imputed as the mid-point between two detected R peaks via the MindWare HRV software program. The rater also checked the data to ensure respiration rates measured via impedance cardiography (Ernst et al. 1999) were within the expected physiological range (.15–.40 Hz) for this age group to allow the accurate quantification of RSA (i.e., heart rate variation associated with spontaneous respiration). Data outside of this range were not included in the study and accounts for 0.48 % of the total data collected (4 min).
The MindWare mobile units also allow for the simultaneous collection of motion data using an accelerometer. Accelerometer data was collected in the x, y, and z planes and summed for a minute-by-minute total that was then averaged for each participant at baseline, and during each of the four TSST conditions.
Of the total collected data, we discarded 4.3 % due to excessive motion artifact that interfered with the identification of R peaks. All data included for further statistical analysis had a confirmed respiratory frequency within the expected physiological range of .15–.40 Hz, a clear ECG waveform, including an R peak, and spanned at least ten respiratory cycles.
Statistical Analysis Plan
We performed all statistical analyses using SPSS Version 22.0 (IBM Corp, 2013) Independent t tests were used to examine group differences in demographic and clinical variables (see Table 1). These include pubertal stage, verbal and performance IQ, SRS and SCQ scores. An independent samples t test was also conducted on accelerometer data at baseline to test for differences in motion; we also conducted a repeated measures ANOVA with motion during each TSST period as the dependent variable to test for potential effects of diagnosis on differences in movement.
We used Levene’s Test for Homogeneity of Variance to test for significant effects of diagnosis on within-group RSA data variability.
To test for baseline group differences in RSA, a univariate ANOVA was used with baseline RSA as the dependent variable and pubertal status as a covariate of no interest. To test for effects of diagnosis on RSA during the TSST stressor, we conducted a two-way (diagnosis: ASD, TD) linear mixed effects ANOVA with the four TSST periods of RSA (preparation, speech, serial subtraction and debriefing) as repeated dependent variables and pubertal status as a covariate of no interest. Because of similar variance in RSA measures across time, we used a compound symmetry structure model to reduce the number of parameters in our model.
To test for effects of diagnosis on RSA decrease during the TSST, we calculated residualized change scores for minimum mean RSA value during the TSST, adjusted for the baseline RSA value using regression analysis to account for the initial value (Wilder 1962), a common practice used in the study of RSA (e.g. Heilman et al. 2008). A univariate ANOVA was then performed to test for significant main effects of diagnosis on these residualized change scores, with pubertal status as a covariate of no interest.
Pearson bivariate correlation analyses were performed to assess for symptom correlates of RSA change using the residualized RSA change scores and the SRS, SCQ total t-scores, as well as the Internalizing Symptoms, Social Problems, and Anxiety Problems t-score subscales of the CBCL in the ASD group only.
Results
Preliminary descriptive statistics showed significant differences in variability between males and females, driven largely by the female ASD group (see Supplemental Table). Because we were underpowered to detect significant main effects of diagnosis by sex on RSA, we opted to remove the eight females and proceed with a sample of adolescent males only. The final sample contained 13 TD participants and 21 ASD participants (Table 1).
Independent samples t tests revealed no significant pubertal stage, verbal, or performance IQ differences between groups. There was a significant difference between groups in SCQ and SRS total scores. There were no significant differences in motion at baseline (t = −0.809, p = 0.424) or during the TSST (F = 2.88, p = 0.100) between diagnostic groups.
Levene’s Test revealed a significant difference in baseline RSA variability between groups (F = 4.174, p = 0.049, Fig. 2), with the ASD group showing more variability than TD. There were no significant between-group differences in variability during the other portions of the TSST (all p values >0.05). Thus, are findings are in partial support of hypothesis one.
Fig. 2.
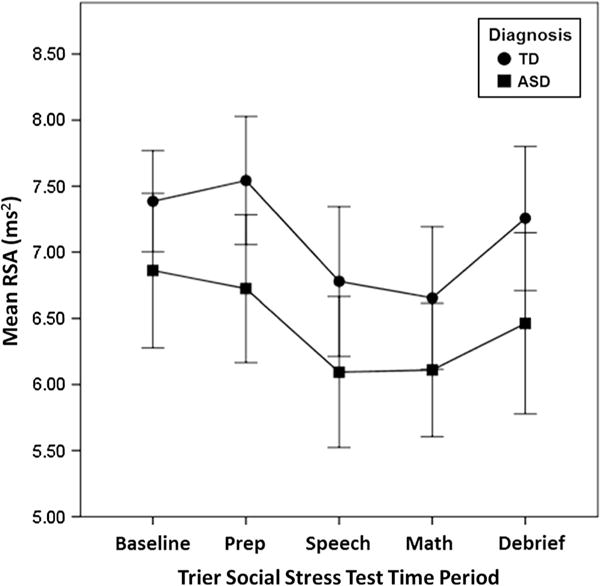
Main effect of diagnosis on respiratory sinus arrhythmia. ASD autism spectrum disorder, TD typical development, RSA respiratory sinus arrhythmia. Error bars represent ±2 standard error
Univariate ANOVA showed a significant main effect of group on baseline RSA (F = 4.480, p = 0.043, η2 < .20), (Table 2), with lower RSA in the ASD group. Linear mixed effects ANOVA demonstrated a significant main effect of group on RSA during the TSST (F(1, 32) = 4.217, p = 0.048), with lower RSA values in the ASD group. There was a main effect of time period (F(3, 96) = 9.877, p < 0.0001), but no diagnosis by TSST time period interaction effects (F(3, 96) = 0.337, p = 0.798) (Table 3).
Table 2.
Between-group baseline RSA findings
| Mean (SD)
|
Levene’s test for equality of variances
|
Univariate ANOVA
|
|||||
|---|---|---|---|---|---|---|---|
| TD (n = 13) | ASD (n = 21) | F value | p value | F value | p value | Effect size | |
| Baseline RSA | 7.50 (0.70) | 6.77 (1.23) | 4.17 | 0.049 | 4.48 | 0.043 | <0.20 |
Table 3.
Between-group descriptive statistics for RSA during the Trier Social Stress Test
| Mean RSA | SD | ||
|---|---|---|---|
| Prep. period | TD | 7.54 | 0.88 |
| ASD | 6.67 | 1.17 | |
| Speech period | TD | 6.78 | 1.02 |
| ASD | 6.08 | 1.24 | |
| Math period | TD | 6.65 | 0.97 |
| ASD | 6.08 | 1.10 | |
| Debriefing period | TD | 7.26 | 0.98 |
| ASD | 6.47 | 1.44 |
Mixed effects model ANOVA with main effect of diagnosis (F = 5.072, p = 0.031) and time (F = 11.419, p < 0.0001)
Univariate ANOVA showed no significant between-group differences in residualized RSA change scores (F = 0.020, p = 0.889, η2 < .20). Thus, our primary hypotheses were only partially supported; there were between group differences in baseline RSA and RSA during the TSST, but not in RSA decrease during the TSST (Fig. 2).
In the ASD group, there was a significant negative correlation between baseline RSA and SRS total score (r = −0.346, p = 0.045, Fig. 3a) and the CBCL Internalizing Symptoms subscale (r = −0.550, p = 0.012, Fig. 3b), but not SCQ total score (r = −0.205, p = 0.386), the CBCL Social Problems subscale (r = −0.330, p = 0.155), the CBCL Anxiety Problems subscale (r = −0.393, p = 0.086, Table 4).
Fig. 3.
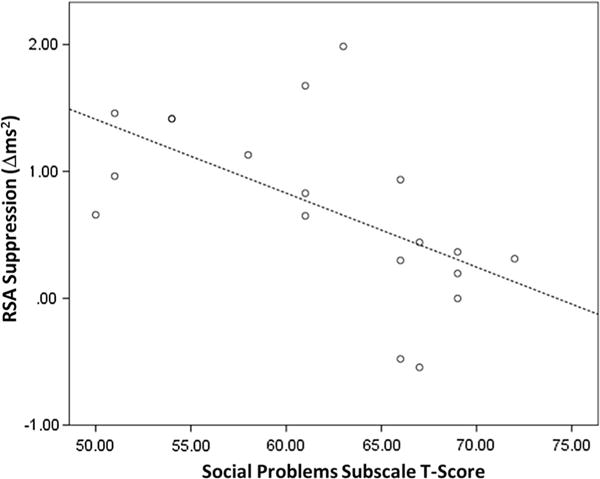
Correlation between residualized RSA change scores and the social problems subscale of the childhood behavioral checklist in autism spectrum disorder. RSA respiratory sinus arrhythmia, r = −0.585, p = 0.008
Table 4.
Pearson correlation analyses between parental report measures and RSA change scores in ASD group
| SCQ total score | SRS total t-score | CBCL internalizing symptoms subscale | CBCL social problems subscale | CBCL anxiety problems subscale | |
|---|---|---|---|---|---|
| Residualized RSA change score | −.018 | −0.255 | −0.146 | −0.586** | −0.011 |
SCQ social communication questionnaire, SRS Social Responsiveness Scale, CBCL Childhood Behavior Checklist
p < 0.05;
p < 0.01
There was a significant negative correlation between the residualized RSA change score, i.e., our metric of the maximal RSA response during the stressor, and the CBCL Social Problems t-score in the ASD group (r = −0.586, p = 0.007), indicating that less change in RSA from baseline during the TSST was correlated with greater severity of social problems in the ASD group (Fig. 3). There was no significant relationship between RSA change scores and the other CBCL subscales of interest, the Internalizing Symptoms subscale (r = −0.146, p = 0.540) and Anxiety Problems subscale (r = −0.011, p = 0.964), or the measures of ASD symptom severity, the SCQ (r = −0.018, r = 0.941) and the SRS (r = −0.255, p = 0.278, Table 4).
Discussion
In the present study of adolescent males, we aimed to determine the physiologic stress response to social judgment via measurement of RSA. Participants with ASD showed lower RSA values compared to TD males, both at baseline and during the TSST. These initial findings present preliminary evidence for altered overall physiological self-regulation in ASD adolescent males. Post hoc investigation of relationships between parental reports of social problems, but not ASD symptoms, anxiety, or internalizing problems, were correlated with RSA change scores in response to the TSST in the ASD group. Taken together, these preliminary findings suggest that social problems in ASD may be linked to the RSA response to social stress. This view of the role of RSA changes as a facilitator of flexible social engagement would be in line with the growing literature showing relationships between greater RSA decreases and emotional and behavioral regulation (Calkins and Dedmon 2000; Butler et al. 2006; Miller et al. 2013; Cui et al. 2015). However, one study in TD children comparing the relative contributions of baseline RSA and RSA change to social behavior found a significant relationship between observed empathy and high baseline RSA but not RSA decreases, which was more closely associated with negative emotionality (Liew et al. 2011). While another recent study found a significant relationship between baseline RSA and empathy that was mediated by effortful control in a sample of young TD children (Taylor et al. 2015). Future studies with larger samples should assess for potential contributions of anxiety versus social symptoms to RSA decreases in ASD versus TD adolescents.
The lack of a group by time period interaction effect suggests that there is not a between-group responsivity difference in ASD and TD adolescents at a specific time point during the TSST, but rather, an overall RSA reduction in the ASD group across all time points. Furthermore, the lack of a group difference finding in RSA change during the TSST suggests that the onset of the social stressor was not a primary between-group differentiator of PNS function, but that the ASD group overall shows reduced physiological self-regulation that was present at baseline and persisted throughout the task and during the debriefing period. Our findings confirm and extend previous work highlighting the relationship between baseline RSA and various measures of social ability and prosocial behavior in both clinical and community samples (D’Antono et al. 2005; Hastings et al. 2008; Diamond et al. 2011; Hinnant and El-Sheikh 2009; Beauchaine et al. 2013; Graziano and Derefinko 2013; Patriquin et al. 2013; Sulik et al. 2013; Taylor et al. 2015).
In this study, we found a preliminary correlation between maximal RSA change following exposure to the social stressor and social problems. Porges’ Polyvagal Theory (1995 Porges’ Polyvagal Theory (2007) argues that high baseline RSA denotes greater ability to react flexibly and adaptively to the changing demands of the environment and that high baseline RSA supports adaptive coping in the face of environmental challenges. The literature regarding PNS differences in ASD has been varied, with some reporting no differences between ASD and TD groups at baseline (Daluwatte et al. 2012; Levine et al. 2012; Watson et al. 2012; Toichi and Kamio 2003; Althaus et al. 1999) and some showing decreased basal PNS function in ASD (Bal et al. 2010; Vaughan Van Hecke et al. 2009; Ming et al. 2005). Consistently, no studies show increases in PNS measures in ASD compared TD groups. Our findings of a relationship between RSA decreases and social problems as indexed by the CBCL indicate that perhaps some of the variation in the ASD RSA literature may be due to heterogeneity within the ASD diagnosis, It appears that careful clinical characterization with larger clinical samples is important for determining the relationship between physiological regulation and social judgment in this population.
To date, there have been only a few studies using the TSST to investigate psychophysiological response to social judgment in ASD. For example, one study by Levine et al. (2012) included pre-pubertal children with ASD and employed the TSST-C, a version adapted for children (Buske-Kirschbaum et al. 1997). The authors did not find significant differences in HRV between TD and ASD groups, but there was a non-significant overall reduction in RSA in the ASD group at baseline and during all portions of the TSST-C, which parallels the present findings of reduced RSA. Another study used a stress test similar to the TSST, but, instead of a serial subtraction task, asked participants to complete the Rey–Osterrieth Complex Figure task (Mikita et al. 2015). This study measured heart rate as a proxy for stress reactivity to the task, but did not measure HRV or PNS function per se. The authors reported reduced heart rate to the stressor in children with ASD with co-occurring high anxiety compared to children with ASD and low anxiety, which they interpret as “stress induced physiological withdrawal” (Mikita et al. 2015, p. 1124). Finally, Hollocks et al. (2014) employed a variant of the TSST in a mixed sample of ASD children and adolescents with and without comorbid anxiety disorders. The authors examined the ratio of low frequency heart rate fluctuation to high frequency heart rate fluctuation (LF/HF), a measure of the relative contribution of sympathetic arousal to HRV. The authors report group differences in HRV and LF/HF, with the ASD participants with co-occurring anxiety showing a blunted HRV response, but no difference between the ASD participants without anxiety and the TD control group. Hollocks et al. (2014) also report a group effect on heart rate, such that the ASD group with co-occurring anxiety disorders shows heightened heart rate at baseline and throughout the task compared to the ASD group without anxiety and the TD group. Importantly, these studies employed adapted versions of the TSST that ostensibly tax visual attention and memory, and do not use a social peer. Despite these differences from the current protocol, the findings outlined above of blunted ANS measures in ASD are in agreement with the findings in the present study (Levine et al. 2012; Hollocks et al. 2014; Mikita et al. 2015).
We report a significant correlation between social problems and RSA response to social stress, but not anxiety symptoms and RSA response. Previous investigations of children with ASD have reported significant relationships between cardiac-neural measures of stress responsivity and parental reports of anxiety symptoms (Mikita et al. 2015; Guy et al. 2014; Hollocks et al. 2014). To date, the anxiety disorders literature suggests lower RSA at baseline in adults with post traumatic stress and panic disorders, but not specific phobias compared to controls (for review see Friedman 2007; Licht et al. 2009). A recent meta-analytic review of RSA across multiple psychiatric diagnoses, including anxiety disorders, found reduced RSA in adults with psychiatric diagnoses and controls at baseline or in response to a stressor, including unmedicated adults (Alvares et al. 2016). However, other studies in samples of undergraduate students have not found RSA differences between adults with high versus low trait anxiety (Wilhelm et al. 2001), and the only study to employ the TSST in adults with anxiety disorders reported no differences between adults with anxiety disorders and those without (Klumbies et al. 2014). Similar to the adult literature, studies of children with anxiety disorders have suggested lower PNS functioning at baseline (Dieleman et al. 2015). Studies employing emotional regulation or trauma recall tasks in adolescents with anxiety disorders have shown no differences in RSA responsivity (Fisher and Newman 2013; Shenk et al. 2014; Kirsch et al. 2015) or have found differences in female, but not male adolescents (Hastings et al. 2014). To date, there have been no studies employing the TSST in pubertal adolescents with anxiety disorders using RSA as an outcome measure. There is a need for comparison studies of individuals with ASD and co-occurring anxiety disorders and those without, as well as study of the ways that anxiety manifests specifically in ASD versus typical development.
Pubertal development plays an important role in the physiological response to stress, both in terms of the development and stabilization of biological systems underlying the stress response, but also regarding the increased salience and complexity of social stimuli during adolescence, which is defined by the onset of puberty (Sisk and Foster 2004; Gunnar et al. 2009). Studies of RSA in the general population confirm the importance of adolescent development physiologically and psychosocially (Woodall and Matthews 1993). There is also evidence implicating sex differences in RSA, including greater variability in adult females than adult males (Snieder et al. 2007), which is consistent with the findings of the present study of adolescents. Future studies should clarify the contributions of sex steroid hormones during puberty to ANS function, reactivity, and stabilization by carefully accounting for sex steroid levels and, in females, the role of menstrual cycle in physiological regulation and reactivity. Studies in the general adult population have indicated that there are both sex differences and menstrual cycle effects on the physiological response to social evaluative threat. Specifically, studies have shown greater heart rate response and subjective feelings of anxiety to the TSST during the luteal phase, suggesting that progesterone levels may mediate some aspects of the stress response (Gordon and Girdler 2014; Childs et al. 2010). Furthermore, for adolescent girls with ASD, social deficits may become more pertinent in adolescence than they are for male adolescents because social relationships may be more challenging, demanding, and complex for adolescent girls than they are for males (McLennan et al. 1993). In one study of age- and IQ-matched adolescent boys and girls with ASD, there were not significant differences in core symptoms of autism, but the parents of girls with ASD were more likely to report significant social deficits via the CBCL than the parents of boys. Parents of the girls in this study were also less likely to report peer friendships than the parents of boys. The authors interpret these findings by suggesting that the parents of girls may have higher expectations for social competence than the parents of boys, and that peers in the school environment also mirror these heightened expectations (Holtmann et al. 2007). Particularly in ASD, where females are more likely to be misdiagnosed or diagnosed later in life (Russell et al. 2011), there is a clear need for research that focuses on the experiences and needs of adolescent girls with ASD, especially as they are related to understanding and improving social abilities.
Despite the careful characterization of the sample in the present study, there are some limitations. To best address peak physiological response to stress, we subtracted each participant’s lowest mean RSA during the TSST from baseline RSA. We chose this approach because it allows us to measure group differences in the maximal stress response to the social stress paradigm. However, this approach does not allow us to address potential individual differences in the timing of RSA decreases. Future studies should assess for possible differences in the timing of biophysiological responses to social stress in ASD versus TD. The findings in the present study may not extend to other subpopulations within ASD. For example, we were limited in our ability to address RSA findings in adolescent females due to greater variability within the ASD cohort. Future studies that include a large sample of adolescent females are warranted to elucidate the mechanisms contributing to physiological variability in adolescent females with ASD. Future studies should also assess RSA in a more naturalistic, non-laboratory setting, as TD and ASD adolescents may differ in the degree to which they find the laboratory environment stressful (Corbett et al. 2012). Baseline measures of RSA taken at home in a familiar environment could provide an interesting comparison to RSA data collected in the lab. This study was part of a larger project that also investigated salivary cortisol response to the TSST. As such, we excluded participants taking medications known to interfere with HPA axis function during recruitment. Exclusion of adolescents taking medications such as atypical antipsychotics could potentially have biased our sample. Furthermore, because of the cognitively demanding nature of the TSST paradigm, we were not able to include individuals with intellectual disabilities, which also limits the generalizability of our findings. There was a ten point difference in total estimated IQ between our ASD and TD samples, although this difference was not significant and both groups had mean IQs that feel within the normal range, the lack of a statistical difference could be due to a relatively small sample size. Possible relationships between IQ variation and RSA in ASD are interesting and warrant further study. Finally, although our findings were statistically significant, our effect sizes are relatively small. This is due to our relatively small sample size; future studies with larger clinical samples are needed.
To our knowledge, this is the first study examining RSA in adolescent males with ASD. The current investigation contributes to the growing literature on the biopsychosocial correlates of ASD in adolescence. The majority of previous work focusing on children has found reduced RSA in response to social stressors in ASD (Benevides and Lane 2015). Although we did not find the hypothesized difference in variability between groups during the stressor, there was an overall group effect of reduced RSA in the ASD group, providing preliminary evidence that reduced PNS activity in children with ASD persists into adolescence. Lower PNS activity in ASD may contribute to some of the social challenges observed in ASD, including reduced social engagement and flexibility. These findings highlight the importance of autonomic arousal in ASD and the relationship of autonomic arousal to internalizing symptoms that may cause significant distress for individuals with ASD and their families. Furthermore, there is preliminary evidence that an ASD intervention targeting auditory processing is associated with an increase in RSA and a corresponding improvement in sensory processing, suggesting that (a) RSA is plastic and responsive to treatment and (b) that alterations in autonomic system functioning may have broad implications for a host of ASD symptoms associated with social engagement (Porges et al. 2013). Longitudinal studies that investigate the developmental effects of puberty on RSA are critical for an improved understanding of biopsychosocial development in ASD. Future studies should also compare across types of potential stressors to determine if one type of stressor (i.e., social vs. nonsocial stress) may induce greater physiological effects in ASD.
Supplementary Material
Acknowledgments
The authors would like to thank the adolescents and their families who participated in this study. This manuscript was prepared from the first author’s doctoral dissertation.
Funding This study was funded by NIMH R01 MH085717.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10803-016-2842-1) contains supplementary material, which is available to authorized users.
Author Contributions EKE participated in the design of the study, collection, analysis, and interpretation of data and drafting of the manuscript. RMJ participated in the analysis and interpretation of data and drafting of the manuscript. BAC conceived of the study, particpated in the collection and interpretation of the data, and drafting of the manuscript.
Conflict of interest All authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- Achenbach TM, Howell CT, Quay HC, Conners CK. National survey of problems and competencies among four- to sixteen-year-olds: Parents’ reports for normative and clinical samples. Monographs of the Society for Research in Child Development. 1991;56(3):1–131. [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Althaus M, Mulder LJ, Mulder G, Aarnoudse CC, Minderaa RB. Cardiac adaptivity to attention-demanding tasks in children with a pervasive developmental disorder not otherwise specified (PDD-NOS) Biological Psychiatry. 1999;46(6):799–809. doi: 10.1016/s0006-3223(98)00374-6. [DOI] [PubMed] [Google Scholar]
- Althaus M, Van Roon AM, Mulder LJ, Mulder G, Aarnoudse CC, Minderaa RB. Autonomic response patterns observed during the performance of an attention-demanding task in two groups of children with autistic-type difficulties in social adjustment. Psychophysiology. 2004;41(6):893–904. doi: 10.1111/j.1469-8986.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. Journal of psychiatry and neuroscience. 2016;41(2):89–104. doi: 10.1503/jpn.140217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders. 2010;40(3):358–370. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology. 2015;3:43–47. doi: 10.1016/j.copsyc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J, Reid MJ, Webster-Stratton C. Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. Journal of Consulting and Clinical Psychology. 2013;81(3):481–493. doi: 10.1037/a0032302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides TW, Lane SJ. A review of cardiac autonomic measures: Considerations for examination of physiological response in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45(2):560–575. doi: 10.1007/s10803-013-1971-z. [DOI] [PubMed] [Google Scholar]
- Bolte S, Westerwald E, Holtmann M, Freitag C, Poustka F. Autistic traits and autism spectrum disorders: the clinical validity of two measures presuming a continuum of social communication skills. Journal of Autism and Developmental Disorders. 2011;41(1):66–72. doi: 10.1007/s10803-010-1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BB, Eicher SA, Petrie S. The importance of peer group (“crowd”) affiliation in adolescence. Journal of Adolescence. 1986;9(1):73–96. doi: 10.1016/s0140-1971(86)80029-x. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43(6):612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Canivez GL, Konold TR, Collins JM, Wilson G. Construct validity of the Wechsler Abbreviated Scale of Intelligence and Wide Range Intelligence Test: Convergent and structural validity. School Psychology Quarterly. 2009;24(4):252–265. [Google Scholar]
- Chandler S, Charman T, Baird G, Simonoff E, Loucas T, Meldrum D, Pickles A. Validationg of the social communication questionnaire in a population cohort of children with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(10):1324–1332. doi: 10.1097/chi.0b013e31812f7d8d. [DOI] [PubMed] [Google Scholar]
- Charlot L, Deutsch CK, Albert A, Hunt A, Connor DF, McIlvane WJ. Mood and anxiety symptoms in psychiatric inpatients with autism spectrum disorder and depression. Journal of Mental Health Research in Intellectual Disabilities. 2008;1(4):238–253. doi: 10.1080/19315860802313947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Van Dam NT, de Wit H. Effects of acute progesterone administration upon responses to acute psychosocial stress in men. Experimental and Clinical Psychopharmacology. 2010;18(1):78–86. doi: 10.1037/a0018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Hinden BR, Gerhardt CA. Adolescent development: Pathways and processes of risk and resilience. Annual Review of Psychology. 1995;46:265–293. doi: 10.1146/annurev.ps.46.020195.001405. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Corbett BA, Schupp CW, Lanni KE. Comparing biobehavioral profiles across two social stress paradigms in children with and without autism spectrum disorders. Molecular Autism. 2012;3(1):13. doi: 10.1186/2040-2392-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Simon D, Ryan N, Mendoza S. Elevated cortisol during play is associated with age and social engagement in children with autism. Molecular Autism. 2010;1(1):13. doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Morris AS, Harrist AW, Larzelere RE, Criss MM, Houltberg BJ. Adolescent RSA responses during an anger discussion task: Relations to emotion regulation and adjustment. Emotion. 2015;15(3):360–372. doi: 10.1037/emo0000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daluwatte C, Miles JH, Yao G. Simultaneously measured pupillary light reflex and heart rate variability in healthy children. Physiological Measurement. 2012;33(6):1043–1052. doi: 10.1088/0967-3334/33/6/1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antono B, Moskowitz DS, Miners C, Archambault J. Gender and communal trait differences in the relations among social behaviour, affect arousal, and cardiac autonomic control. Journal of Behavioral Medicine. 2005;28(3):267–279. doi: 10.1007/s10865-005-4663-0. [DOI] [PubMed] [Google Scholar]
- De Meersman RE. Aging as a modulator of respiratory sinus arrhythmia. Journal of Gerontology. 1993;48(2):B74–B78. doi: 10.1093/geronj/48.2.b74. [DOI] [PubMed] [Google Scholar]
- Diamond LM, Hicks AM, Otter-Henderson KD. Individual differences in vagal regulation moderate associations between daily affect and daily couple interactions. Personality and Social Psychology Bulletin. 2011;37(6):731–744. doi: 10.1177/0146167211400620. [DOI] [PubMed] [Google Scholar]
- Dieleman GC, Huizink AC, Tulen JHM, Utens EMWJ, Creemers HE, van der Ende J, Verhulst FC. Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology. 2015;51:135–150. doi: 10.1016/j.psyneuen.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Dubin AH, Lieberman-Betz R, Michele Lease A. Investigation of individual factors associated with anxiety in youth with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2015;45(9):2947–2960. doi: 10.1007/s10803-015-2458-x. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst JM, Litvack DA, Lozano DL, Cacioppo JT, Berntson GG. Impedance pneumography: Noise as signal in impedance cardiography. Psychophysiology. 1999;36:333–338. doi: 10.1017/s0048577299981003. [DOI] [PubMed] [Google Scholar]
- Espelage DL, Bosworth K, Simon TR. Short-term stability and prospective correlates of bullying in middle-school students: An examination of potential demographic, psychosocial, and environmental influences. Violence and Victims. 2001;16(4):411–426. [PubMed] [Google Scholar]
- Fisher AJ, Newman MG. Heart rate and autonomic response to stress after experimental induction of worry versus relaxation in healthy, high-worry, and generalized anxiety disorder individuals. Biological Psychology. 2013;93:65–74. doi: 10.1016/j.biopsycho.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Foley P, Kirschbaum C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neuroscience and Biobehavioral Reviews. 2010;35(1):91–96. doi: 10.1016/j.neubiorev.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Gjevik E, Eldevik S, Fjæran-Granum T, Sponheim E. Kiddie-SADS reveals high rates of DSM-IV disorders in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2011;41(6):761–769. doi: 10.1007/s10803-010-1095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS. Mechanisms underlying hemodynamic and neuroendocrine stress reactivity at different phases of the menstrual cycle. Psychophysiology. 2014;51(4):309–318. doi: 10.1111/psyp.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34(10):1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology. 2013;94(1):22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy L, Souders M, Bradstreet L, DeLussey C, Herrington JD. Brief report: Emotion regulation and respiratory sinus arrhythmia in autism spectrum disorder. Journal of Autism and Developmental Disorders. 2014;44(10):2614–2620. doi: 10.1007/s10803-014-2124-8. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Klimes-Dougan B, Kendziora KT, Brand A, Zahn-Waxler C. Regulating sadness and fear from outside and within: Mothers’ emotion socialization and adolescents’ parasympathetic regulation predict the development of internalizing difficulties. Development and Psychopathology. 2014;26:1369–1384. doi: 10.1017/S0954579414001084. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, Sullivan C. Applying the polyvagal theory to children’s emotion regulation: Social context, socialization, and adjustment. Biological Psychology. 2008;79(3):299–306. doi: 10.1016/j.biopsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Taylor CB. The relationship between agoraphobia symptoms and panic disorder in a nonclinical sample of adolescents. Psychological Medicine. 2003;33(4):733–738. doi: 10.1017/s0033291702006955. [DOI] [PubMed] [Google Scholar]
- Heilman KJ, Bal E, Bazhenova OV, Sorokin Y, Perlman SB, Hanley MC, Porges SW. Physiological responses to social and physical challenges in children: Quantifying mechanisms supporting social engagement and mobilization behaviors. Developmental Psychobiology. 2008;50(2):171–182. doi: 10.1002/dev.20257. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Children’s externalizing and internalizing symptoms over time: the role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology. 2009;37(8):1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Hollocks MJ, Howlin P, Papadopoulos AS, Khondoker M, Simonoff E. Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology. 2014;46:32–45. doi: 10.1016/j.psyneuen.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Bölte S, Poustka F. Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology. Developmental Medicine and Child Neurology. 2007;49(5):361–366. doi: 10.1111/j.1469-8749.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Hutt C, Hutt SJ, Lee D, Ounsted C. Arousal and childhood autism. Nature. 1964;204:908–909. doi: 10.1038/204908a0. [DOI] [PubMed] [Google Scholar]
- Jarrin DC, McGrath JJ, Poirier P, Séguin L, Tremblay RE, Montplaisir JY, Séguin JR. Short-term heart rate variability in a population-based sample of 10-year-old children. Pediatric Cardiology. 2015;36(1):41–48. doi: 10.1007/s00246-014-0962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Mack ME. Does aging differentially reduce heart rate variability related to respiration? Experimental Aging Research. 1984;10(1):19–23. doi: 10.1080/03610738408258536. [DOI] [PubMed] [Google Scholar]
- Kirsch V, Wilhelm FH, Goldbeck L. Psychophysiological characteristics of pediatric posttraumatic stress disorder during script-driven traumatic imagery. European Journal of Psychotraumatology. 2015;6:25471. doi: 10.3402/ejpt.v6.25471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klumbies E, Braeuer D, Hoyer J, Kirschbaum C. The reaction to social stress in social phobia: Discordance between physiological and subjective parameters. PLoS ONE. 2014;9:e105670. doi: 10.1371/journal.pone.0105670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewski M, Alifier M, Bochen D, Urban M. Heart rate turbulence in children—age and heart rate relationships. Pediatric Research. 2007;62(6):710–714. doi: 10.1203/PDR.0b013e3181598836. [DOI] [PubMed] [Google Scholar]
- Kushki A, Brian J, Dupuis A, Anagnostou E. Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism. 2014;5:39. doi: 10.1186/2040-2392-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TP, Sheinkopf SJ, Pescosolido M, Rodino A, Elia G, Lester B. physiologic arousal to social stress in children with autism spectrum disorders: A pilot study. Research in Autism Spectrum Disorders. 2012;6(1):177–183. doi: 10.1016/j.rasd.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, van Dyck R, Penninx BW. Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA) Psychosomatic Medicine. 2009;71:508–518. doi: 10.1097/PSY.0b013e3181a292a6. [DOI] [PubMed] [Google Scholar]
- Liew J, Eisenberg N, Spinrad TL, Eggum ND, Haugen RG, Kupfer A, Baham ME. Physiological regulation and fearfulness as predictors of young children’s empathy-related reactions. Social Development. 2011;20(1):111–113. doi: 10.1111/j.1467-9507.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Petkova E, Hus V, Gan W, Lu F, Martin DM, Risi S. A multisite study of the clinical diagnosis of different autism spectrum disorders. Archives of General Psychiatry. 2012;69(3):306–313. doi: 10.1001/archgenpsychiatry.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JW. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosomatic Medicine. 1968;30(5):576–607. [PubMed] [Google Scholar]
- Matson JL, Nebel-Schwalm MS. Comorbid psychopathology with autism spectrum disorder in children: An overview. Research in Developmental Disabilities. 2007;28(4):341–352. doi: 10.1016/j.ridd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Mazzone L, Ruta L, Reale L. Psychiatric comorbidities in asperger syndrome and high functioning autism: Diagnostic challenges. Annals of General Psychiatry. 2012;11(1):16. doi: 10.1186/1744-859X-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic–pituitary–adrenal function, and programming of adult learning and memory. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(5):756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McLennan JD, Lord C, Schopler E. Sex differences in higher functioning people with autism. Journal of Autism and Developmental Disorders. 1993;23(2):217–227. doi: 10.1007/BF01046216. [DOI] [PubMed] [Google Scholar]
- Merchan-Naranjo J, Mayoral M, Rapado-Castro M, Llorente C, Boada L, Arango C, Parellada M. Estimation of the intelligence quotient using Wechsler Intelligence Scales in children and adolescents with asperger syndrome. Journal of Autism and Developmental Disorders. 2011;42(1):116–122. doi: 10.1007/s10803-011-1219-8. [DOI] [PubMed] [Google Scholar]
- Mikita N, Hollocks MJ, Papadopoulos AS, Aslani A, Harrison S, Leibenluft E, Stringaris A. Irritability in boys with autism spectrum disorders: An investigation of physiological reactivity. Journal of Child Psychology and Psychiatry. 2015;56(10):1118–1126. doi: 10.1111/jcpp.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Chocol C, Nuselovici JN, Utendale WT, Simard M, Hastings PD. Children’s dynamic RSA change during anger and its relations with parenting, temperament, and control of aggression. Biological Psychology. 2013;92(2):417–425. doi: 10.1016/j.biopsycho.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain and Development. 2005;27(7):509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Turner CA, Goldstein G. The application of short forms of the Wechsler Intelligence Scales in adults and children with high-functioning autism. Journal of Autism and Developmental Disorders. 2005;35(1):45–52. doi: 10.1007/s10803-004-1030-x. [DOI] [PubMed] [Google Scholar]
- Moskowitz LJ, Mulder E, Walsh CE, McLaughlin DM, Zarcone JR, Proudfit GH, Carr EG. A multimethod assessment of anxiety and problem behavior in children with autism spectrum disorders and intellectual disability. American Journal on Intellectual and Developmental Disabilities. 2013;118(6):419–434. doi: 10.1352/1944.7558.118.6.419. [DOI] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Holdrinet I, Meesters C. Comorbid anxiety symptoms in children with pervasive developmental disorders. Journal of Anxiety Disorders. 1998;12(4):387–393. doi: 10.1016/s0887-6185(98)00022-x. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Scarpa A, Friedman BH, Porges SW. Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology. 2013;55(2):101–112. doi: 10.1002/dev.21002. [DOI] [PubMed] [Google Scholar]
- Pellecchia M, Connell JE, Kerns CM, Xie M, Marcus SC, Mandell DS. Child characteristics associated with outcome for children with autism in a school-based behavioral intervention. Autism. 2015 doi: 10.1177/1362361315577518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pico-Alfonso MA, Mastorci F, Ceresini G, Ceda GP, Manghi M, Pino O, Sgoifo A. Acute psychosocial challenge and cardiac autonomic response in women: The role of estrogens, corticosteroids, and behavioral coping styles. Psychoneuroendocrinology. 2007;32(5):451–463. doi: 10.1016/j.psyneuen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Massari F, Mastropasqua F, Fioretti A, Guida P, Colombo R, Rizzon P. Age effect on phase relations between respiratory oscillations of the RR interval and systolic pressure. Pacing and Clinical Electrophysiology. 2000;23(5):847–853. doi: 10.1111/j.1540-8159.2000.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Vagal tone: A physiologic marker of stress vulnerability. Pediatrics. 1992;90(3 Pt 2):498–504. [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behavior: A polyvagal perspective. Infant and Child Development. 2011;20(1):106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Macellaio M, Stanfill SD, McCue K, Lewis GF, Harden ER, Heilman KJ. Respiratory sinus arrhythmia and auditory processing in autism: Modifiable deficits of an integrated social engagement system? International Journal of Psychophysiology. 2013;88(3):261–270. doi: 10.1016/j.ijpsycho.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman GI, Masten AS, Coatsworth JD, Tellegen A. Salient and emerging developmental tasks in the transition to adulthood. Child Development. 2004;75(1):123–133. doi: 10.1111/j.1467-8624.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- Russell G, Steer C, Golding J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Social Psychiatry and Psychiatric Epidemiology. 2011;46(12):1283–1293. doi: 10.1007/s00127-010-0294-z. [DOI] [PubMed] [Google Scholar]
- Rutter M. Categories, dimensions, and the mental health of children and adolescents. Annals of the New York Academy of Sciences. 2003;1008:11–21. doi: 10.1196/annals.1301.002. [DOI] [PubMed] [Google Scholar]
- Schaaf RC, Benevides TW, Leiby BE, Sendecki JA. Autonomic dysregulation during sensory stimulation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45(2):461–472. doi: 10.1007/s10803-013-1924-6. [DOI] [PubMed] [Google Scholar]
- Schroeder J, Weiss J, Bebko J. CBCL profiles of children and adolescents with asperger syndrome: A review and pilot study. Journal on Developmental Disabilities. 2011;17(1):26–37. [Google Scholar]
- Shahrestani S, Stewart EM, Quintana DS, Hickie IB, Guastella AJ. Heart rate variability during adolescent and adult social interactions: A meta-analysis. Biological Psychology. 2015;105:43–50. doi: 10.1016/j.biopsycho.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Shenk CE, Putnam FW, Rausch JR, Peugh JL, Noll JG. A longitudinal study of several potential mediators of the relationship between child maltreatment and posttraumatic stress disorder symptoms. Development and Psychopathology. 2014;26:81–91. doi: 10.1017/S0954579413000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Snieder H, van Doornen LJ, Boomsma DI, Thayer JF. Sex differences and heritability of two indices of heart rate dynamics: A twin study. Twin Research and Human Genetics. 2007;10(2):364–372. doi: 10.1375/twin.10.2.364. [DOI] [PubMed] [Google Scholar]
- Sulik MJ, Eisenberg N, Silva KM, Spinrad TL, Kupfer A. Respiratory sinus arrhythmia, shyness, and effortful control in preschool-age children. Biological Psychology. 2013;92(2):241–248. doi: 10.1016/j.biopsycho.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Borres M, Thulesius O, Tamai H, Ericson MO, Lindblad LE. Blood pressure and cardiovascular autonomic function in healthy children and adolescents. The Journal of Pediatrics. 2000;137(1):63–67. doi: 10.1067/mpd.2000.108098. [DOI] [PubMed] [Google Scholar]
- Taylor ZE, Eisenberg N, Spinrad TL. Respiratory sinus arrhythmia, effortful control, and parenting as predictors of children’s sympathy across early childhood. Developmental Psychology. 2015;51(1):17–25. doi: 10.1037/a0038189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toichi M, Kamio Y. Paradoxical autonomic response to mental tasks in autism. Journal of Autism and Developmental Disorders. 2003;33:417–426. doi: 10.1023/a:1025062812374. [DOI] [PubMed] [Google Scholar]
- van Steensel FJ, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clinical Child and Family Psychology Review. 2011;14(3):302–317. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, Porges SW. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development. 2009;80(4):1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Watson LR, Roberts JE, Baranek GT, Mandulak KC, Dalton JC. Behavioral and physiological responses to child-directed speech of children with autism spectrum disorders or typical development. Journal of Autism and Developmental Disorders. 2012;42(8):1616–1629. doi: 10.1007/s10803-011-1401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilder J. Basimetric approach (law of initial value) to biological rhythms. Annals of the New York Academy of Sciences. 1962;98(1):1211–1220. doi: 10.1111/j.1749-6632.1962.tb30629.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Kochar AS, Roth WT, Gross JJ. Social anxiety and response to touch: Incongruence between self-evaluative and physiological reactions. Biological Psychology. 2001;58(3):181–202. doi: 10.1016/s0301-0511(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Witwer AN, Lecavalier L. Autism screening tools: an evaluation of the social communication questionnaire and the developmental behavior checklist–autism screening algorithm. Journal of Intellectual and Developmental Disability. 2007;32(3):179–187. doi: 10.1080/13668250701604776. [DOI] [PubMed] [Google Scholar]
- Woodall KL, Matthews KA. Changes in and stability of hostile characteristics: Results from a 4-year longitudinal study of children. Journal of Personality and Social Psychology. 1993;64(3):491–499. doi: 10.1037//0022-3514.64.3.491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


