Abstract
Proteins secreted by pathogens during host colonization largely determine the outcome of pathogen-host interactions and are commonly called ‘effectors’. In fungal plant pathogens, coordinated transcriptional up-regulation of effector genes is a key feature of pathogenesis and effectors are often encoded in genomic regions with distinct repeat content, histone code and rate of evolution. In the tomato pathogen Fusarium oxysporum f. sp. lycopersici (Fol), effector genes reside on one of four accessory chromosomes, known as the ‘pathogenicity’ chromosome, which can be exchanged between strains through horizontal transfer. The three other accessory chromosomes in the Fol reference strain may also be important for virulence towards tomato. Expression of effector genes in Fol is highly up-regulated upon infection and requires Sge1, a transcription factor encoded on the core genome. Interestingly, the pathogenicity chromosome itself contains 13 predicted transcription factor genes and for all except one, there is a homolog on the core genome. We determined DNA binding specificity for nine transcription factors using oligonucleotide arrays. The binding sites for homologous transcription factors were highly similar, suggesting that extensive neofunctionalization of DNA binding specificity has not occurred. Several DNA binding sites are enriched on accessory chromosomes, and expression of FTF1, its core homolog FTF2 and SGE1 from a constitutive promoter can induce expression of effector genes. The DNA binding sites of only these three transcription factors are enriched among genes up-regulated during infection. We further show that Ftf1, Ftf2 and Sge1 can activate transcription from their binding sites in yeast. RNAseq analysis revealed that in strains with constitutive expression of FTF1, FTF2 or SGE1, expression of a similar set of plant-responsive genes on the pathogenicity chromosome is induced, including most effector genes. We conclude that the Fol pathogenicity chromosome may be partially transcriptionally autonomous, but there are also extensive transcriptional connections between core and accessory chromosomes.
Author Summary
Eukaryotic genomes are organised. Genomic regions may differ in spatial organisation, chromatin condensation, rate of evolution, GC content, gene expression and transposon density. Many plant pathogenic fungi maintain genomic subcompartments containing specialized genes to facilitate host colonisation. These genes, for example effector genes, show concerted transcriptional up-regulation during infection. An extreme case is the tomato pathogen Fusarium oxysporum f. sp. lycopersici, which carries accessory chromosomes, of which one encodes all effector genes and can be transferred horizontally between strains. We investigated the transcriptional connections between this accessory chromosome and the core genome, particularly with respect to effector gene expression. Several of the transcription factors encoded on this accessory chromosome bind to motifs enriched on accessory chromosomes, suggesting the accessory chromosomes may be partially transcriptionally independent. Only one of these, Ftf1, can induce the expression of effector genes and binds to a motif that is enriched in their promoters. Also Sge1 –a conserved regulator of fungal lifestyle switches and required for infection–can activate the expression of effector genes. Both transcription factors induce a largely overlapping set of genes, including many of the host-induced genes on the accessory chromosome including the effector genes. This demonstrates the existence of extensive transcriptional connections between accessory and core chromosomes.
Introduction
Plant pathogenic fungi are genetically adapted to infect their host plant, but are also in a constant arms race with that host to stay virulent. For this, pathogens need to allow accelerated evolution of pathogenicity-related genes, without affecting the function of housekeeping genes. One possibility is to spatially separate these different functional groups of genes into subgenomic compartments with different rates of evolution. One of the fastest evolving determinants of pathogenicity is the effector repertoire. Definitions vary, but a typical effector is a small, secreted protein that affects the interaction between the pathogen and its host. In many plant pathogenic fungi effector genes indeed reside in specific genomic regions, generally distinguished by one or more of the following characteristics: lineage-specific or accessory, rich in transposable elements, a different GC content and/or codon bias from the rest of the genome, depleted for housekeeping genes and associated with particular chromatin modifications [1–3]. Accumulating evidence suggests that these genomic environments evolve more rapidly than the rest of the genome and facilitate adaptation [2,4,5]. In addition, in several fungi these types of regions—or parts thereof—have been shown or suggested to transfer horizontally between different strains or even species [6–11].
Another hallmark of effector genes is a plant specific expression pattern [12–14]. How coordinated expression of effector genes is regulated and how this is related to the specific genomic environment of these genes is poorly understood. For some effector genes it has been shown that their genomic environment is key for regulated expression, through histone modifications [1,15]. On the other hand, a small number of transcription factors required for effector gene expression has been identified. In Ustilago maydis the heterodimer bE/bW, the forkhead transcription factor Fox1, and the zinc finger transcription factors Rbf1 and Mzr1 are involved in transcriptional regulation of pathogenicity-related genes and/or effector genes [16–19]. In Leptosphaeria maculans and Stagnospora nodorum, homologs of StuA, a bHLH (basic helix-loop-helix) type of transcription factor, regulate expression of several effector genes [20,21]. Most is known about the role of Wor1 orthologs in effector gene expression. Wor1 is a conserved fungal transcription factor from Candida albicans, with a WOPR type of DNA binding domain [22]. In plant pathogenic fungi from the genus Fusarium (putative) effector genes and/or secondary metabolite gene clusters are regulated by an ortholog of this transcription factor: F. oxysporum f. sp. lycopersici (Sge1), F. graminearum (Fgp1), F. verticillioides (FvSge1) and F. fujikuroi (FfSge1) [23–26]. Also in the plant pathogenic fungi Botrytis cinerea (Reg1), Verticillium dahliae (VdSge1), Cladosporium fulvum (CfWor1), Zymoseptoria tritici (ZtWor1), Ustilago maydis (UmRos1) and Magnaporthe oryzae (MoGti1) deletion of the gene for this transcription factor (partially) perturbs expression of effector genes [27–32]. Mutant strains deleted for this gene are mostly non-pathogenic (except Δffsge1), although for CfWOR1 this may be a secondary effect of a developmental phenotype. In C. albicans, Wor1 was originally discovered as a ‘master regulator’ of the morphological switch from white to opaque cells. Also in Saccharomyces cerevisiae and Histoplasma capsulatum the Wor1 orthologs (Mit1 and Ryp1, respectively) regulate a morphological transition, which, both in C. albicans and H. capsulatum, is associated with differences in virulence towards humans [33]. This led to the idea that Wor1 orthologs in plant pathogenic fungi are also master regulators of a lifestyle switch, from saprotrophic to pathogenic.
In Fusarium oxysporum f. sp. lycopersici (Fol), the causal agent of Fusarium wilt in tomato, effector genes (called SIX genes for ‘Secreted In Xylem’) reside on an accessory chromosome that can be transferred horizontally between strains. Upon receipt of this accessory chromosome of Fol, a non-pathogenic strain can acquire pathogenicity towards tomato [7]. This means that effector gene expression must be ensured in different genomic environments (i.e. in the original strain and in the recipient strain). Two different but not mutually exclusive strategies to ensure effector gene expression are: i) to rely on conserved transcription factors encoded on the core genome or ii) to encode the transcription factors necessary for effector gene expression on the accessory chromosome itself. As mentioned above, Fol effector gene expression requires the presence of the core-encoded conserved transcription factor Sge1 [26]. However, also on the accessory chromosome transcription factors are encoded, 13 in total [34]. One of these transcription factor genes, FTF1, is associated with highly virulent strains of F. oxysporum f. sp. phaseoli and is up-regulated during infection [35,36]. In addition, FTF1 is present in three variants on the Fol pathogenicity chromosome and all three genes are located close to single or small groups of effector genes [34].
Although the pathogenicity chromosome of Fol is transcriptionally connected to the core genome via Sge1, the presence of numerous transcription factor genes on the chromosome itself suggests that this accessory chromosome might be transcriptionally semi-autonomous. To see whether this may be the case, we investigated the role of the transcription factors encoded on the pathogenicity chromosome of Fol in effector gene expression.
Results
The pathogenicity chromosome of Fol encodes nine transcription factor gene families, of which four comprise multiple genes on accessory chromosomes
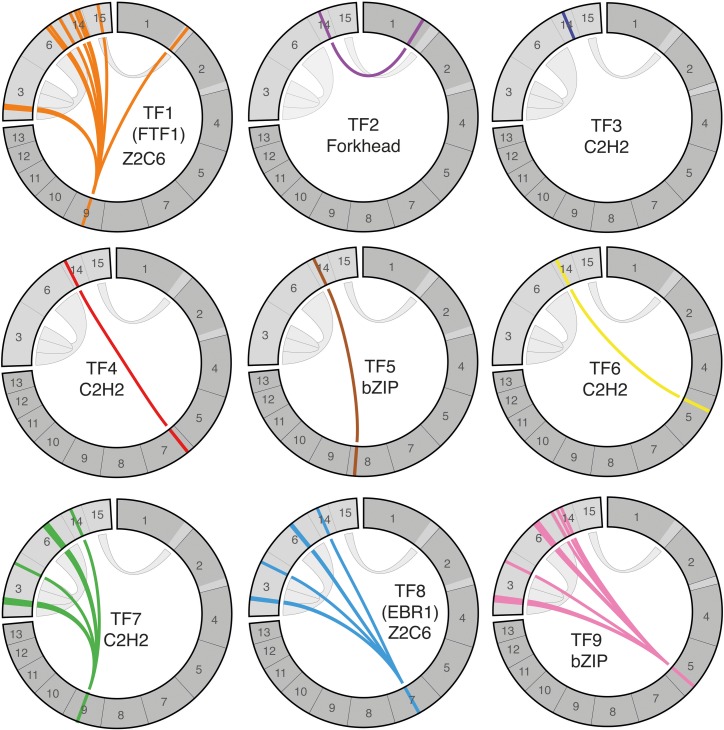
To see if the pathogenicity chromosome of Fol (chromosome 14 in reference strain Fol4287) may be transcriptionally autonomous, we inventoried the transcription factors it encodes. We found 13 predicted transcription factor genes that cluster into nine families. The transcription factor gene families were numbered TF1 to TF9 and include one homolog of EBR1 (TF8) and three homologs of FTF1 (TF1) (Fig 1, S1 Data: tab ‘TF table’) [35,37]. Most gene families encode proteins containing zinc finger DNA binding domains; four are Cys2His2 zinc finger DNA binding domains (Tf3, Tf4, Tf6 and Tf7) and two are Zn(2)Cys(6) zinc finger DNA binding domains (Tf1 and Tf8). Additionally, there are two gene families encoding transcription factors with a basic leucine zipper (bZIP) DNA binding domain (Tf5 and Tf9) and one gene family encoding forkhead transcription factors (Tf2). All transcription factor genes on the pathogenicity chromosome have a homolog on the core genome, except TF3 (Fig 1). Four of the transcription factor gene families have also expanded on other accessory chromosomes of Fol4287 (TF1, TF7, TF8 and TF9).
Fig 1. Transcription factor genes on the pathogenicity chromosome have homologs on the core genome and on other accessory chromosomes.
Schematic representation of the chromosomes of Fol and the position of transcription factor genes on the pathogenicity chromosome (chromosome 14) and their homologs on other chromosomes. All accessory regions are depicted in light grey and core chromosomes in dark grey. Note that small accessory regions are attached to core chromosomes 1 and 2. Grey ribbons indicate duplicated genomic regions. Transcription factor genes are indicated with colored bars, each transcription factor gene family is represented in a separate circos plot.
The F. oxysporum species complex encompasses many different formae speciales (ff.spp.), each with a specific plant host and specific accessory genomic material. All transcription factor gene families (except TF3) have one core- and one or more accessory-encoded homologs in other ff. spp. of F. oxysporum investigated. (Fig 2, S1 Fig, S1 Data:tab ‘TF table’). From here on, we refer to accessory homologs as aTF and to core homologs as cTF. The other Fusarium species analysed (F. solani, F. graminearum and F. verticillioides) each have only one homolog of most of the transcription factors; the expansion we see on the accessory regions in F. oxysporum has not occurred. Regardless of the forma specialis, core-encoded transcription factors form one clade (Fig 2, S1 Fig, indicated with a grey bar) and show little sequence divergence. In general, the F. verticillioides homologs are closest to this clade, consistent with the species phylogeny. Accessory chromosome-encoded homologs show more divergence, both within and between strains, and are also more diverse in sequence than the core encoded homologs in F. oxysporum and F. verticillioides. This suggests that either i) the expansion and divergence of the transcription factor genes on the accessory chromosomes is older than the separation between F. oxysporum and F. verticillioides, similar to what was suggested for accessory genes in general [7], or ii) the rate of evolution is accelerated in the accessory regions compared to the core genome, or iii) a combination of the two.
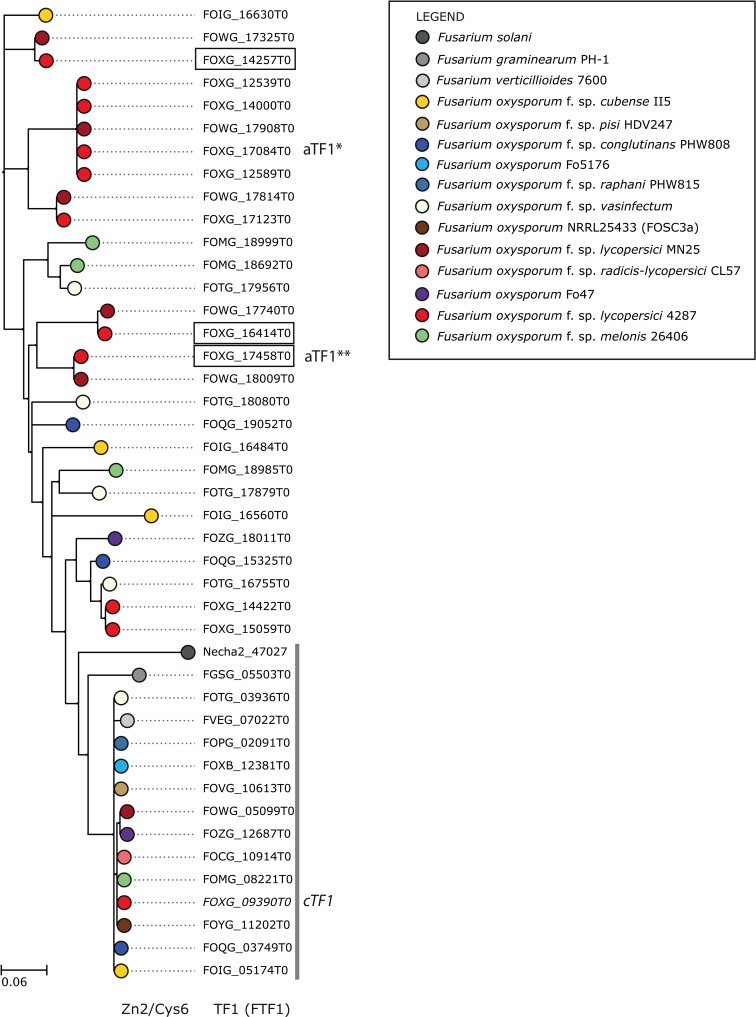
Fig 2. Transcription factors encoded on accessory chromosomes of F. oxysporum have diverged more than core-encoded transcription factors between Fusarium species.
Phylogenetic tree of the Tf1 (Ftf1) family based on protein sequence alignments, including homologs in Fol, other F. oxysporum ff. spp. and other Fusarium species. The aTf1 homologs encoded on the pathogenicity chromosome in Fol are boxed, and the core homolog (cTf1) is written in italics. Cloned genes are indicated with aTF1 or cTF1. aTF1*: short version of aTF1, aTF1**: long version of aTF1. A grey bar indicates the clade with Fol, Fv, Fg and Fs core-encoded homologs. Phylogenetic trees of the other transcription factor families are presented in S1 Fig.
DNA binding specificity is conserved between homologous transcription factors
As transcription factor gene families have expanded and diverged on the accessory chromosomes, we wanted to investigate whether some of these genes may have neofunctionalized. To see if homologous core- and accessory-encoded transcription factors regulate different target genes, we set out to determine the DNA binding sites of each transcription factor encoded on the pathogenicity chromosome and its core-encoded homolog.
Transcription factor coding sequences were cloned from cDNA, in vitro translated as a GST-fusion and hybridised with two different oligo arrays (called HK & ME) [38]. Binding enrichments were inferred for each possible 8-mer. Of 13 transcription factors the cDNA could be amplified and cloned. For aTf1, aTf8 and aTf9, cloning of any of the homologs on the pathogenicity chromosome was unsuccessful. Possibly this was due to both low transcript levels and the presence of homologous transcripts; only hybrid PCR products were amplified. However, a partial cDNA encoding the predicted DNA binding domain from another aTf1 homolog was obtained (FOXG_17084 on accessory chromosome 6). Tf1 homologs separate in two groups based on the length of the coding sequence. The first group (~3200 bp, we refer to this as the longer coding sequence) include the core homolog, two homologs on the pathogenicity chromosome and two identical genes on other accessory chromosomes. The remaining aTF1 genes are shorter (~2800 bp), because they have a more downstream startcodon and a more upstream stopcodon (S2 Fig) [35]. All Tf1 homologs have highly similar DNA binding domains (S3 Fig). The cloned aTF1 cDNA (FOXG_17084) has a short coding sequence.
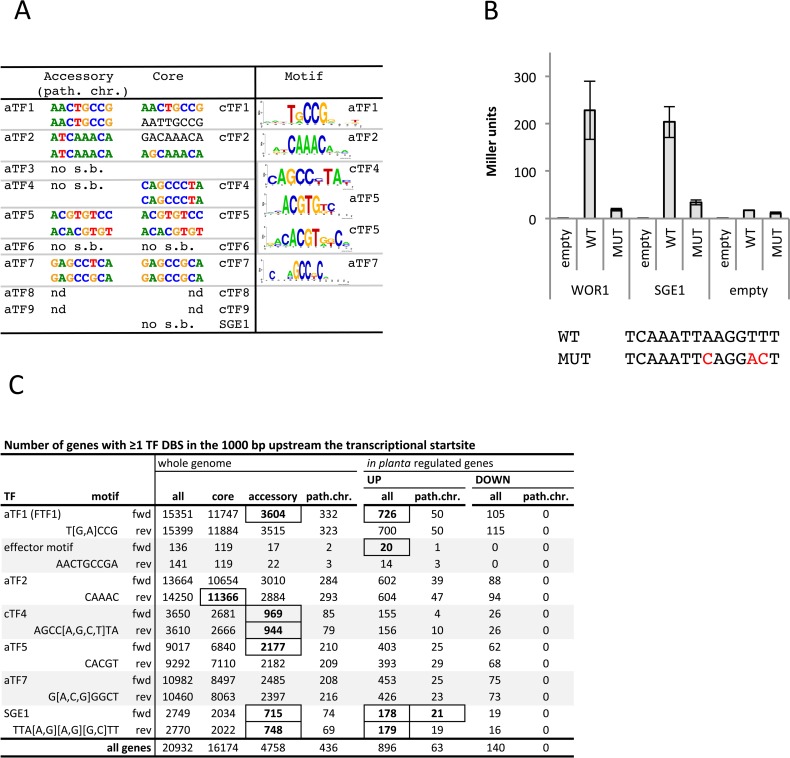
For nine of the cloned transcription factors a reliable DNA binding site could be inferred from one or both arrays (Fig 3A, S1 Data: tab ‘DNA binding assay’). In all cases both arrays yielded similar top 8-mers. For the remaining four transcription factors no significant enrichment was found. The DNA binding sites of homologous transcription factors were the same or very similar in all four families for which both homologs yielded a DNA binding site, indicating that no diversification in recognition specificity has occurred. The DNA binding site of aTf2 and its homolog cTf2 overlap with the consensus DNA binding site of forkhead transcription factors (RYMAAYA) [39]. The aTF5/cTF5 DNA binding site is almost palindromic, which is in accordance with the dimeric structure of leucine zippers. Tf1 has a Gal4-like Zn(2)Cys(6) DNA binding domain, which in Gal4 binds as a dimer to the CGGN11CCG consensus sequence [40]. The DNA binding site of aTf1 and cTf1 (TRCCG) overlaps with half of this consensus. Interestingly, the aTf1 DNA binding site overlaps with a motif found earlier to be enriched in the promoters of effector genes: aacTGCCGa [34]. Note that the top 8-mer for both aTf1 and cTf1 is perfectly contained in this motif (Fig 3A, S1 Data: tab ‘DNA binding assay’).
Fig 3. DNA binding motifs are not diversified within transcription factor families, but some motifs are enriched on accessory chromosomes.
A) DNA binding motifs determined by DNA binding arrays for several accessory and core transcription factor pairs. Accessory transcription factors were cloned from the pathogenicity chromosome in all cases except aTF1; this transcription factor was cloned from another accessory chromosome. Of transcription factors with a reliable DNA binding site on one or two arrays, the top 8-mers for both arrays are given (in color: significant enriched binding [E-score > 0.45], in grey, E-score < 0.45). For each transcription factor: upper 8-mer: HK array, lower 8-mer: ME array. For transcription factors with significantly enriched binding to ten 8-mers or more, a motif was constructed of all 8-mers with significant binding (right hand side of the figure). B) Sge1 can transcriptionally activate an UAS-less CYC1 promoter via the presence of the Wor1 DNA binding site. Activation is lost when the Wor1 DNA binding site is absent or mutated (lower panel). Activation assays were performed in duplicate, error bars represent standard deviation. C) The number of genes with one or more transcription factor DNA binding site (TF DBS) in the promoter, for the complete genome, for a subgenome (core, accessory or pathogenicity chromosome), and for a subset of genes (up- or down-regulated during infection). Boxes indicate a significant enrichment (P value < 0.01 after Bonferroni correction). The motif found in effector promoters earlier (aacTGCCGa) and overlapping with the Tf1 DNA binding site has been included.
We attempted to determine the DNA binding site of Sge1 in a similar way, but no significant 8-mer enrichments were detected. Sge1 has an unusual fungi-specific WOPR type DNA binding domain. The DNA binding site and protein crystal structure of several homologs of Sge1 have been resolved [33,41–43]. The amino acids interacting with DNA are highly conserved among Wor1/Sge1 homologs from different fungi [23,27,29,30,41,43,44]. Consistent with this, the DNA binding sites of Sge1 orthologs in Saccharomyces cerevisiae (Mit1) and the filamentous fungus Histoplasma capsulatum (Ryp1) are the same as for Wor1 [33].
To test whether Sge1 can also bind to the Wor1-DNA binding site, we have adopted an in vivo transcriptional activation assay developed previously [42]. In short, Wor1 or Sge1 is produced constitutively in yeast together with a reporter construct consisting of the Wor1-DNA binding site and an UAS-less CYC1 promoter fused to the lacZ gene. The two SGE1 homologs in yeast (YEL007 or Mit1 and YHR177) are deleted from the yeast strain to avoid potential cross-activation. We found that Sge1 can induce the reporter gene to the same level as Wor1, and this ability is lost when the Wor1 DNA-binding site is mutated (Fig 3B). This confirms that Sge1 binds the same DNA sequence as its orthologs and is a transcriptional activator.
Accessory chromosomes are enriched for DNA binding sites of some transcription factors encoded on the pathogenicity chromosome
Determination of the DNA binding site of several transcription factors showed that DNA binding specificity has not or hardly diverged between accessory-encoded and core-encoded transcription factors. However, if accessory-encoded transcription factor homologs fulfill an important role in transcriptional regulation of the accessory chromosomes, target genes of these transcription factors could be enriched there. To test this, the number of genes with minimally one, two or three binding sites in 1 kb upstream of the annotated transcriptional start site was counted (Fusarium Comparative Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/), annotation 3) (S1 Data: tab ‘promoter DBS’). Because some of the sequence motifs contain relatively little information we found many occurrences throughout the genome. Nevertheless, for several DNA binding sites (of aTf1, cTf4 and aTf5) significant enrichment (hypergeometric test, P value < 0.01 after Bonferroni correction) was found on accessory chromosomes, and for the aTf2 DNA binding site enrichment was found on the core genome (Fig 3C, S1 Data: tab ‘promoter DBS’). Specific enrichment of DNA binding sites on the pathogenicity chromosome, however, was not detected. In an effort to reduce noise levels for the smaller sequence motifs we also looked at multiple occurrences, and indeed the observed enrichments were also present under these criteria. Interestingly, also the Sge1 DNA binding site is enriched on the accessory chromosomes. To test whether the observed enrichments are specific for promoter regions, we performed the same test for the 1000 bp downstream the ATG of each gene. We found no DNA binding site enrichments in the coding regions, except for the aTf1 DNA binding site, which was found enriched both in the promoter and in the coding regions of accessory genes (S4A and S4B Fig, S1 Data: tab ‘ORF DBS’).
Since the pathogenicity chromosome and the effectors encoded on it are important for the infection of tomato plants, enrichment of DNA binding sites among genes that are up-regulated during infection and those that are down-regulated was also tested [45]. Only the aTf1 and the Sge1 DNA binding sites were significantly enriched among up-regulated genes, and none of the DNA binding sites was enriched among genes down-regulated during infection (Fig 3C, S1 Data).
Taken together, this statistical analysis suggests that several transcription factor gene families on the pathogenicity chromosome may be involved in regulating targets on accessory chromosomes. In addition, aTf1 and/or cTf1 and Sge1 in particular may target genes involved in pathogenicity.
Expression of aTF1, cTF1 or SGE1 from a constitutive promoter induces SIX1 expression
The effectors encoded on the pathogenicity chromosome are important determinants for pathogenicity and are under strict transcriptional regulation [26,46–50]. To determine whether transcription factors encoded on the pathogenicity chromosome can induce effector gene expression, strains ectopically expressing these transcription factors from a constitutive promoter—from hereon called ‘overexpressors’–were generated and tested for their ability to induce effector gene expression. For seven out of the nine transcription factor families encoded on the pathogenicity chromosome we were able to make constructs with the constitutive FEM1 promoter [51] to drive expression (cloning of aTF8 and aTF9 was unsuccessful). Three aTF1 homologs are present on the pathogenicity chromosome and one of these was selected for overexpression (FOXG_17458, long coding sequence). Also one ‘short’ aTF1 gene from another accessory region was chosen for overexpression (FOXG_17084), the same gene that was used for the DNA binding site determination.
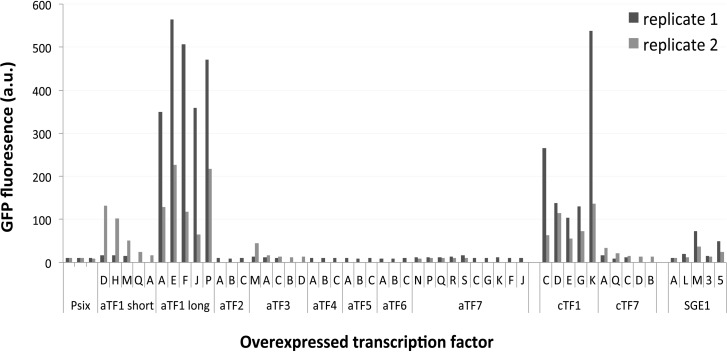
To facilitate screening for the induction of effector gene expression, transcription factor overexpression constructs were transformed into a strain carrying a reporter gene. In this Fol strain, the reporter (GFP) replaces the ORF of the effector gene SIX1 –a representative plant-induced effector gene–so that it is expressed in locus from the SIX1 promoter [46]. For each transcription factor, 11 to 23 independent transformants were inspected for increase of GFP expression using fluorescence microscopy (S5A Fig). GFP signals varied between transformants with the same construct. The three to ten transformants that appeared to respond most strongly to each transcription factor were used to quantify GFP levels spectrophotometrically (Fig 4). Most accessory transcription factors tested did not affect SIX1 expression when expressed from the FEM1 promoter. In contrast, the long version of aTF1 very strongly induced SIX1 expression, and the shorter version conferred some induction. Also SGE1, tested in the same way, can induce SIX1 expression, albeit to a lesser extent than aTF1. To confirm overexpression of the transcription factor genes in the transformants that showed no GFP induction, transcript levels were determined with quantitative RT-PCR. In all cases an increase of transcription factor gene expression compared to the background strain was observed in at least one out of three transformants tested (S5B Fig).
Fig 4. Expression of aTF1 (FTF1), cTF1 (FTF2) or SGE1 from a constitutive promoter activates the SIX1 promoter.
Quantitative measurement of GFP fluorescence in strains expressing transcription factor genes from the FEM1 promoter. For each transcription factor fluorescence of 2*10^6 spores was measured in several independent transformants, as indicated with letters or numbers. Psix1 is the background strain (Fol007 with the Psix1GFP reporter construct).
Some of the strains expressing SGE1 or the long version of aTF1 from the FEM1 promoter showed slight growth retardation, and strains overexpressing aTF7 (FOXG_14275) consistently grow slower on both rich and minimal medium (S5C Fig). The same transformants were tested for their ability to infect tomato plants. Only aTF7 overexpression altered this ability; aTF7-overexpressors were less virulent, which may be explained by their retarded growth (S5D Fig).
To see whether the phenotypes caused by aTF1 or aTF7 overexpression could also be induced by overexpression of their core homologs, strains expressing cTF1 (FOXG_09390, long gene model) or cTF7 (FOXG_17774) from the FEM1 promoter were generated and tested as described above (S5 Fig, Fig 4). cTF1 can activate the SIX1 promoter almost to the levels of the long version of aTF1, and cTF7 overexpressors have a similar growth retardation and reduced virulence phenotype as those overexpressing aTF7. This suggests a similar function for core and accessory homologs at least in these two cases.
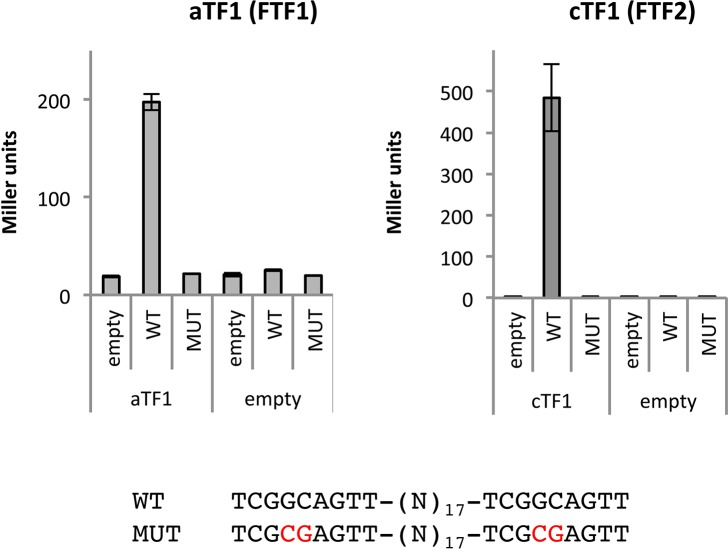
aTf1 and cTf1 are transcriptional activators
Since expression of aTF1 and cTF1 from the FEM1 promoter was shown to potently induce the SIX1 promoter, and the SIX1 promoter contains several aTf1 DNA binding sites, we proceeded to test the results of the DNA binding array in an independent system. For this we used the aTF1 homolog (FOXG_17458) used for overexpression and cTF1 in the in vivo transcriptional activation assay described above for Sge1. In this case, a short fragment was cloned from the promoter of the SIX1 effector gene. This sequence includes two Tf1-DNA binding motifs that overlap with the previously identified motif aacTGCCGa and are separated by 17 basepairs. We also constructed a version with mutated Tf1 DNA-binding sites (two basepair substitutions; Fig 5, lower panel). Both aTf1 and cTf1 are able to activate transcription from the construct with the wild type SIX1 promoter fragment, but not the mutated fragment (Fig 5). This assay was performed in the yeast strain lacking SGE1 homologs (YEL007 or Mit1 and YHR177), showing that DNA binding and transcriptional activation by aTf1 and cTf1 does not require (a homolog of) Sge1.
Fig 5. aTf1 and cTf1 (Ftf1 and Ftf2) are transcriptional activators.
aTf1 and cTf1 can transcriptionally activate an UAS-less CYC1 promoter via the presence of two Tf1 DNA binding sites. Activation is lost when the Tf1 DNA binding site is absent or mutated (lower panel). Activation assays were performed in duplicate, error bars represent the standard deviation.
Transcriptome analysis of aTF1, cTF1 or SGE1 overexpressors
We found that only SGE1, aTF1 and its core homolog cTF1 bind to motifs enriched in the promoters of plant-induced genes and genes on accessory chromosomes. Also, expression of any of these three transcription factor genes from the FEM1 promoter induces effector (SIX1) gene expression. Of both SGE1 and cTF1 a role in pathogenicity has been established previously: the Fol Δsge1 mutant is no longer pathogenic and a Δctf1 (Δftf2) mutant is reduced in pathogenicity [26,45]. Expression of all three transcription factor genes is increased upon infection of tomato plants [26,35,36]. To investigate which genes are regulated by these transcription factors, we compared the transcriptomes of SGE1, aTF1 and cTF1 overexpressors with the transcriptome of the background strain. Overexpressors were preferred over gene deletion strains in this setup because effector genes are only expressed during colonization of the plant, and the plant signal that induces this up-regulation is unknown. Since the deletion mutants are not pathogenic or reduced in pathogenicity, expression cannot reliably be studied in planta with gene deletion strains either. Given that expression levels of SGE1, aTF1 and cTF1 increase during infection, we assume that strains overexpressing either of these transcription factor genes partially mimic the in planta state.
RNA was isolated from two-day old cultures growing in minimal medium. For each transcription factor two independent overexpressors were used, and for each strain three independent biological replicates were sampled. Per sample between 2.8*10^7 and 4.8*10^7 reads were paired-end sequenced (Illumina). Reads were mapped to the reference genome (Fol4287) and differentially expressed genes were called for each pairwise comparison with the background strain, using DEseq software [52]. The different pairs yielded between 396 and 691 differentially expressed genes (S6A Fig).
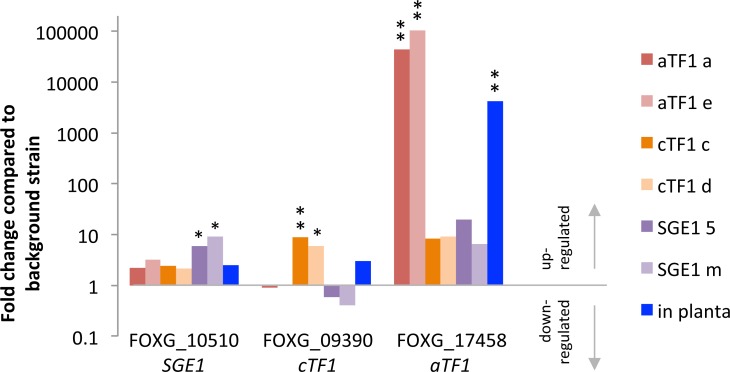
To compare the fold induction of expression of the overexpressed transcription factor genes to their induction during infection, we isolated RNA from Fol-infected tomato plants and from axenic cultures. Both transcriptomes of three biological replicates were sequenced and sequencing reads were mapped to the same annotation of the reference genome as above, using the same parameters. As can be seen in Fig 6, expression of each of the three transcription factor genes is increased in the respective overexpressors as well as during infection. The increase in expression is significant (p<0.05) for all three transcription factors in their respective overexpressors, but during infection only for aTF1. Q-RT-PCR confirmed a significant increase of aTF1 expression in aTF1 overexpressors and during infection, but could not confirm significant differences for cTF1 and SGE1 transcript abundance (S7 Fig).
Fig 6. Fold induction of aTF1 (FTF1), cTF1 (FTF2) and SGE1 expression in overexpressors and during infection.
Fold induction of SGE1, aTF1 and cTF1 transcript abundance during Fol4287-infection (compared to axenic growth of Fol4287) and in their respective overexpressors compared to the background strain (Fol007 Psix1GFP). Red hued bars: aTF1 overexpressors, orange hued bars: cTF1 overexpressors, purple hued bars: SGE1 overexpressors. Blue bars: comparison between Fol4287 in planta and Fol4287 axenic cultures. The wild type strains Fol4287 and Fol007 are very similar [7,50]. One star (*): p<0.05, two stars (**): p<0.01. Note that to calculate fold change, all values of zero were replaced by 0.1 (half of the lowest value in the dataset). This includes the aTF1 levels in cTF1 and SGE1 overexpressors.
Our inability to measure a (significant) increase of cTF1 and SGE1 transcripts above their basal expression level by Q-RT PCR was unexpected. SIX1 (GFP) induction in the independent cTF1 and SGE1 overexpressors may be caused by an only modest increase in transcript levels, as suggested by our RNAseq analysis. For the aTF1 overexpressors, expression of both SIX1 (GFP) and another effector gene (SIX3) does correlate well with aTF1 levels (S8 Fig). Also, only in transformants expressing aTF1, cTF1 or SGE1 from the FEM1 promoter did we ever observe high GFP levels in the Psix1:GFP strain (Fig 4, S5A Fig). Still, we wished to exclude the possibility that the transcriptome changes in the aTF1, cTF1 and SGE1 overexpressors could be due to spontaneous changes unrelated to the transcription factor. We therefore clustered gene expression data of significantly differentially expressed genes for all six overexpressors (S8 Fig). Both aTF1 and both cTF1 overexpressors cluster together, showing that genome wide changes in gene expression in these strains are related to the overexpressed transcription factor. The SGE1 overexpressors, however, do not form a single clade, so we could not link the transcriptional changes in these strains to SGE1 with this approach.
Upstream regions of genes up-regulated in strains overexpressing aTF1, cTF1 or SGE1 are enriched for the respective DNA binding sites
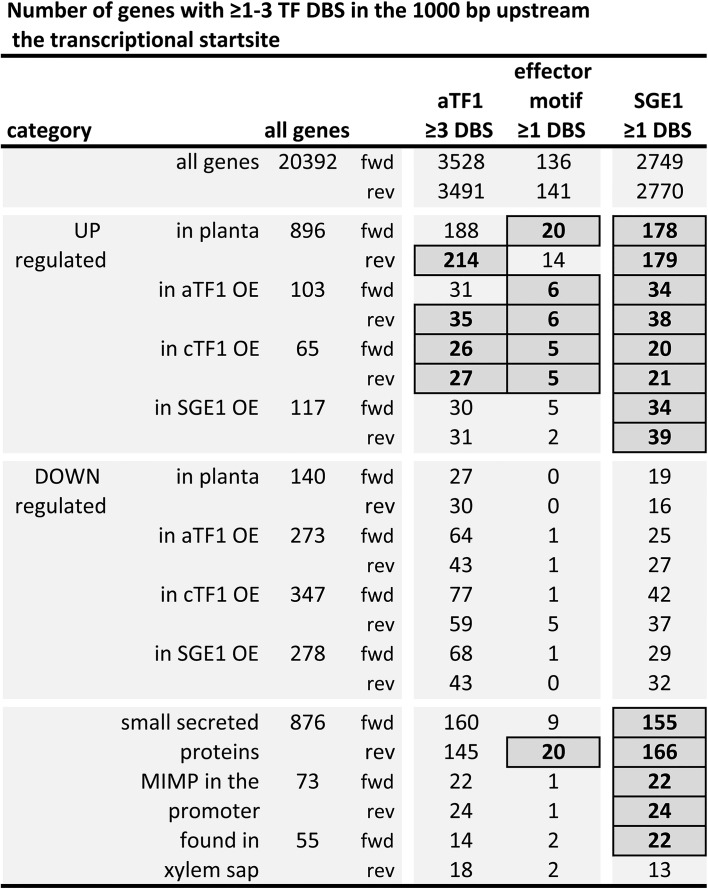
To further test the connection between the selected transcription factors and the changes in the transcriptome, we determined whether the DNA binding site of each transcription factor is enriched in the upstream regions of the genes that are differentially expressed in the aTF1, cTF1 or SGE1 overexpressors. For this we only considered those genes that were significantly up- or down-regulated in both overexpressors of each transcription factor to be consistent transcription factor-specific effects and this selection was used in all further analysis. Under these criteria, each transcription factor up-regulates 65 to 116 genes, and down-regulates 273 to 347 genes, when overexpressed (S6B Fig). We have analysed single occurrence of the Sge1 DNA binding site, triple occurrence of the (relatively short) Tf1 DNA binding site, and single occurrence of the longer motif found in effector gene promoters (that overlaps with the Tf1 DNA binding site) for enrichment in 1kb promoter regions or coding regions (Fig 7, S4C &S4D Fig, S1 Data: tab ‘promoter DBS’ and ‘ORF DBS’). Both the short and the long Tf1 DNA binding sites are significantly and specifically enriched in the promoters of genes up-regulated in both the aTF1 and cTF1 overexpressors, but not in the SGE1 overexpressor. The Sge1 DNA binding site, on the other hand, is specifically enriched in the promoters of SGE1- but also aTF1- and cTF1-up-regulated genes. This links the presence of DNA binding sites to changes in gene expression for all three transcription factors. For none of the down-regulated genes a significant enrichment of DNA binding sites was found, neither for down-regulated genes on the pathogenicity chromosome nor for down-regulated genes on the core chromosomes. This is consistent with the observation that all three transcription factors act as transcriptional activators.
Fig 7. Upstream regions of genes up-regulated in strains overexpressing aTF1 (FTF1), cTF1 (FTF2) or SGE1 are enriched for the respective DNA binding sites.
The number of genes with ≥1 or ≥3 transcription factor DNA binding sites (TF DBS) in the promoter region, for the complete genome, or for a subset of genes. DNA binding sites are i) the Tf1 DNA binding site (≥3 occurrences), ii) the motif found in the promoters of effector genes, overlapping with the Tf1 DNA binding site (≥1 occurrences) and iii) the Sge1 DNA binding site (≥1 occurrences). Subsets of genes are: up- or down-regulated during infection (in planta UP and in planta DOWN), up- or down-regulated in the aTF1, cTF1 or SGE1 overexpressor (aTF1 UP, cTF1 UP, SGE1 UP, aTF1 DOWN, cTF1 DOWN and SGE1 DOWN), genes that code for small secreted proteins, genes with a MIMP (miniature impala) in the promoter and genes that encode proteins that have been found in xylem sap of infected plants [34]. Boxes indicate a significant enrichment (P <0.01 after Bonferroni correction).
Finally, the enrichment of the Tf1 and Sge1 DNA binding site among three other groups of genes was tested (Fig 7). The three groups are: i) genes coding for small secreted proteins; ii) genes with a (partial) miniature impala (MIMP), a non-autonomous transposable element, in the upstream region (up to 2 kb from the ATG—all Fol effector genes are associated with a MIMP, but also other, mainly accessory genes, of which around half is also up-regulated during infection (S6B Fig, [34])); iii) genes of which the protein has been detected in xylem sap of infected plants [34]. The Tf1 DNA binding site is not enriched among these categories, except the long effector motif among small secreted proteins. Remarkably, the Sge1 DNA binding site is enriched in all these categories (Fig 7, lower panel), suggesting that Sge1 may target pathogenicity-associated genes more specifically than aTf1 or cTf1.
Expression of aTF1, cTF1 or SGE1 from the FEM1 promoter induces expression of many plant-responsive genes on the pathogenicity chromosome
We have shown that the genome-wide transcriptional changes in the aTF1 and cTF1 overexpressors are correlated with expression of the respective transcription factor from the FEM1 promoter, while the Sge1 binding site is enriched in upstream regions of genes upregulated in the SGE1 overexpressors and of pathogenicity-associated genes. We now compared the gene sets differentially expressed in the SGE1, aTF1 and cTF1 overexpressors and during infection (Fig 8A and S6B Fig). Strikingly, the majority of the plant-responsive genes on the pathogenicity chromosome is also up-regulated in all three overexpressors, including almost all SIX effector genes (indicated in purple text in Fig 8B). The SIX genes that are not induced are SIX13 in all overexpressors and SIX2 in the SGE1 overexpressors. On the other accessory regions (chromosome 3, 6, 15 plus small regions on chromosome 1 and 2) the overlap between plant responsive genes and Sge1-, aTf1- or cTf1-responsive genes is much smaller. Here a large group of genes is down-regulated in all transformants but not during infection. Of this group many genes are generally weakly expressed. We noticed that lower expression levels of these genes–as observed in the overexpressors–was also found in wild type strains (S9 Fig). This suggests that the apparent down-regulation of expression from this region may not be due to increased abundance of one of the three transcription factors, but rather to general variability in gene expression in this region between transformants. Another transcription factor-unrelated change in expression was observed for a part of the pathogenicity chromosome. In Fig 8B, the top rows of the enlargement of the pathogenicity chromosome show a group of genes up-regulated in one of the cTF1 overexpressors and one of the SGE1 overexpressors. Closer examination showed that these genes are part of a region on supercontig 22 that is generally higher expressed in these two transformants (S10 Fig). Given the regional nature and the lack of correlation to a particular transcription factor, we suspect these differences may be either caused by changes in chromatin state, or by ‘spontaneous’ duplication of these regions. Spontaneous duplications in accessory regions have been reported previously in F. oxysporum [53].
Fig 8. Expression of aTF1 (FTF1), cTF1 (FTF2) or SGE1 from the FEM1 promoter induces many plant-responsive genes on the pathogenicity chromosome.
A) Heatmap of differentially expressed genes during infection or in aTF1, cTF1 or SGE1 overexpressors for the pathogenicity chromosome, other accessory regions and core genome. Displayed is the log2 fold change for each differentially expressed gene (rows), with yellow indicating up-regulation compared to the control (hues cover the log2 fold range from 0 to 5). Blue indicates down-regulation compared to the control (hues cover the log2 fold range from 0 to -5). The log2 fold change values of genes that are not significantly different from the control were set to zero. Each condition is a separate column, with two independent transformants (thus 2 columns) per transcription factor. The order of the rows reflects clustering of similar expression patterns. B) Enlarged heatmap of the pathogenicity chromosome, adorned with gene names: (putative) effector genes are shown in pink, transcription factor genes in blue (aTF1 17458 = FOXG_17458, aTF1 14275 = FOXG_14257, both the longer version of aTF1, aTF1 16414 = FOXG_16414, shorter version of aTF1), other genes are shown in black. SM-47 to SM-56: putative secondary metabolite gene cluster, FOXG_17447 to FOXG_17456.
The majority of all plant-induced genes is located on the core chromosomes, but only a small portion of those is differentially regulated in the aTF1, cTF1 or SGE1 overexpressors. Remarkably, whereas these three transcription factors seem to target the same set of genes on the pathogenicity chromosome, on the core genome the overlap between the genes of which expression is altered by the different transcription factors is much smaller, and each mostly induces a specific set of genes, especially Sge1 (S6A Fig). Still, the genes up-regulated by the transcription factor genes on core chromosomes are enriched for plant-responsive genes (hypergeometric test, P value < 0.05 after Bonferroni correction).
To see which functional categories of genes apart from effector genes are targeted by aTf1, cTf1 and Sge1, a FunCat analysis was performed [54] (S1 Data: tab ‘FungiFun’). Of the genes up-regulated in SGE1 overexpressors, genes in the categories heme binding (including genes coding for cytochrome P450 proteins), catalase reactions and electron transport (including ATPases and oxidoreductases) are overrepresented. Among the predicted functions of the genes induced in the cTF1 overexpressors there is enrichment in secondary metabolism and C-compound and carbohydrate metabolism (including polysaccharide metabolism and protein glycosylation). The set of genes induced by aTf1 is also enriched for genes in secondary metabolism and C-compound and carbohydrate metabolism (including polysaccharide and chitin metabolism as well as pectate lyases).
In the SGE1 overexpressors a peroxisome biogenesis factor (PEX11) is up-regulated. Peroxisome function is required for pathogenicity in Fol [55]. Both aTf1 and cTf1 up-regulate expression of the shorter aTF1 gene on the pathogenicity chromosome (FOXG_16414), although not to the same level as during infection. Apart from this aTF1 homolog, expression of only one other transcription factor gene is significantly altered in any of the overexpressors: aTf1 up-regulates FOXG_04965. Strikingly, this transcription factor is required for pathogenicity [45].
The set of down-regulated genes on the pathogenicity chromosome and the core chromosomes was not significantly enriched for certain categories for aTf1 and cTf1 regulated genes. Sge1-repressed genes were enriched for genes in polysaccharide metabolism and amino saccharide metabolism. Taken together, all three transcription factors influence effector gene expression and secondary metabolism and target genes predicted to affect both the fungal and the plant cell wall (S1 Data: tab ‘FungiFun’).
Expression of aTF1, cTF1 or SGE1 from the FEM1 promoter does not generally increase transcription of transposons
Although very different transcription factors, aTf1/cTf1 and Sge1 induce expression of a large, overlapping set of genes on the pathogenicity chromosome. Together with the observation that some regions may be prone to activation that is not linked to a specific transcription factor gene, this made us wonder whether the pathogenicity chromosome is transcriptionally activated as a whole, rather than gene by gene. The accessory chromosomes are very rich in transposable elements, and we decided to investigate expression of transposable elements as a proxy for chromosome-wide transcriptional activation. Besides, we were interested to see whether general up-regulation of genes on the pathogenicity chromosome (for instance during infection) could jeopardize genome integrity by induction of transposon activity.
One problem, however, is that in our RNAseq analysis the number of transcripts derived from transposable elements was probably highly underestimated, because: i) many transposons are not annotated, ii) reads of identical transposons are distributed over all copies, obscuring activation of individual copies, and iii) reads mapping to more than ten different genomic locations were excluded. To circumvent this, a fasta file was generated where the sequence of each repetitive element, plus all previously annotated transposable elements [34] is present only once. To this file, all sequence reads from the overexpressors as well as reads from infected plant material were mapped (S11 Fig and S6D Fig). We have found no evidence for a general increase of transposable element transcription in the aTF1, cTF1 or SGE1 overexpressors or during plant infection.
Induction of effector gene expression mediated by aTF1 overexpression is Sge1 dependent
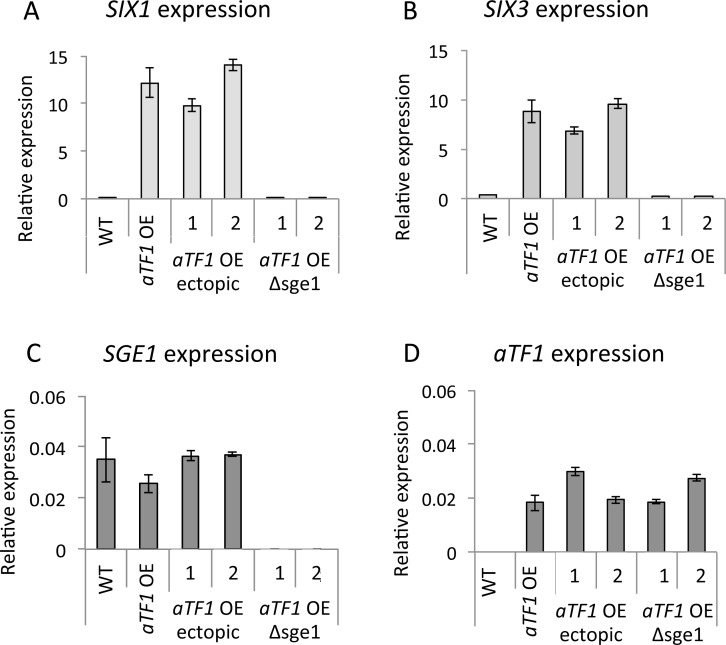
We have shown that expression of Sge1, aTf1 or cTf1 from the FEM1 promoter is correlated with induced expression of a large overlapping set of genes, including effector genes. Whereas aTf1 and cTf1 are homologs and have the same DNA binding specificity, Sge1 is a very different transcription factor, with a strongly conserved, fungal specific DNA binding domain. This raises the question what causes this overlap in transcriptional activation. Expression of for example SIX1 and SIX3 is induced in aTF1, cTF1 and SGE1 overexpressors. However, whereas SIX3 has both Tf1 and Sge1 DNA binding sites present in the promoter, for SIX1 only Tf1 DNA binding sites are present. To test whether the presence of Sge1 is required for aTF1 overexpression-mediated induction of SIX1 and SIX3, we deleted SGE1 in an aTF1 overexpressing strain. Without the presence of SGE1, the expression of the effector genes SIX1 and SIX3 was reduced to low (wild type) levels, while overexpression of aTF1 itself remained unchanged (Fig 9). This shows that Sge1 is required for aTF1 overexpression-mediated activation of effector gene expression, but not necessarily via an Sge1 DNA binding site. Also, deletion of SGE1 resulted in loss of pathogenicity in both WT and in aTF1 overexpressing strains (S12 Fig).
Fig 9. Induction of effector gene expression mediated by aTF1 (FTF1) overexpression is Sge1 dependent.
Relative expression as determined by Q-RT-PCR of SIX1 (A), SIX3 (B), SGE1 (C) and aTF1 (D) in WT, aTF1 overexpressor (aTF1 OE), aTF1 overexpressor transformed with a SGE1 deletion construct, integrated ectopically (aTF1 ectopic, two independent transformants), and aTF1 overexpressor with SGE1 deleted (aTF1 OE Δsge1, two independent transformants). Relative expression is calculated as gene expression/expression of EF1α. Error bars indicate standard deviation.
In summary, we have demonstrated that some pathogenicity chromosome-encoded transcription factors have regulating potential on the accessory chromosomes themselves, and that aTF1 and the core-encoded cTF1 and SGE1 can induce effector gene expression and expression of other plant-responsive genes upon constitutive expression, possibly via direct binding of the transcription factors to these promoters and subsequent transcriptional activation.
Discussion
This study aimed to gain insight in the transcriptional connections between core chromosomes and the accessory ‘pathogenicity’ chromosome in Fol, especially with respect to effector gene expression. We show that expression of the transcription factor genes aTF1 (FTF1, located on the pathogenicity chromosome), cTF1 (FTF2) or SGE1 (both located on the core) from the constitutive FEM1 promoter induces expression of many plant responsive genes located on the pathogenicity chromosome, particularly effector genes. We conclude that the pathogenicity chromosome is transcriptionally partially self-regulating, but not isolated from the core genome.
How do Sge1 and aTf1 work together?
The large overlap in the sets of target genes of aTf1/cTf1 and Sge1 on the pathogenicity chromosome suggests these transcription factors somehow work together. This is substantiated by the observation that aTF1 overexpression-mediated induction of effector gene expression requires Sge1. Overexpression of SGE1 orthologs in F. graminearum, F. verticillioides and F. fujikuroi induces the expression of secondary metabolite genes [23–26]. Apart from SGE1, expression of secondary metabolite gene clusters depends on specialized transcription factors often physically associated with the cluster [56]. Reminiscent of this, SIX effector genes sometimes occur in mini clusters of two or three SIX genes, accompanied by a aTF1 homolog. Genes that are located between or physically close to clustered SIX genes (ORX1, SHH1) are co-induced, both during infection and in the overexpressors.
Transcriptional regulation of secondary metabolite clusters partially takes place on the chromatin level [57,58]. Some observations hint at chromatin-mediated regulation of expression of genes on the pathogenicity chromosome of Fol as well. First, there is the transcription factor unrelated up-regulation of parts of SC22 on the pathogenicity chromosome (in one cTF1 and one SGE1 overexpressor, this study). Second, the BLE resistance gene behind the constitutive GPD promoter located near the SIX1 locus is co-up-regulated in some of the overexpressors (S1 Data, tabs: ‘total mapped reads’ and ‘RPKM’. Finally, physical interactors of the Saccharomyces cerevisiae Sge1 ortholog Mit1 include histones and histone acetyl transferase complexes and are enriched for the GO terms chromosome organization, chromatin assembly or disassembly and chromatin organization (yeastgenome.org). This raises the question whether the large overlap of genes on the pathogenicity chromosome affected by expression of aTF1/ cTF1 and SGE1 from the FEM1 promoter may be caused by a shared influence on chromatin structure. It will be interesting to see if facultative heterochromatin functions as a layer of effector gene expression regulation also in F. oxysporum.
Another explanation for the overlap between the aTF1, cTF1 and SGE1 targets on the pathogenicity chromosome is that Sge1 induces effector gene expression via aTF1/cTF1 or vice versa. Despite the fact that SGE1 has potential aTf1 binding sites in its promoter and one aTF1 (FOXG_17458) has potential Sge1 DNA binding sites in its promoter, neither of the genes is constitutively up-regulated by overexpression of the other. Moreover, Sge1 is still required for effector gene expression when aTF1 is overexpressed. Also, there are clear differences between the different overexpressors, for example SIX11 and SIX2 are induced in aTF1- and cTF1-overexpressors, but not in SGE1-overexpressors. SIX11 and SIX2 indeed have no Sge1 DNA binding site in their promoter. SGE1 is, however, required for the induced expression of SIX2 upon exposure of Fol to tomato cell cultures [26].
Alternatively, Sge1 and aTf1/cTf1 may be simultaneously required at promoters for transcription activation. Sge1 and cTf1 both have basal (non-induced) expression levels, and overexpression of either might cause recruitment of the other (perhaps by direct interaction or through chromatin modification) leading to transcription activation. However, we have shown that Sge1 as well as Tf1 can bind DNA and activate transcription independent of each other. A similar situation has been described for the Sge1 homolog Ryp1 and two velvet-like transcription factors in H. capsulatum [59]. A subset of the genes induced by overexpression of Ryp1 is also induced by overexpression of either of the two velvet-like transcription factors. These shared target genes have DNA binding sites for each transcription factor in their promoter, and the three transcription factors physically interact.
To unravel the question of Sge1/Tf1 co-operation, it will be necessary to determine physical interactions, both between them and with promoters, under basal conditions and upon overexpression, in combination with analysis of chromatin structure at the same promoters.
Are accessory transcription factor homologs redundant?
Next to (nearly) identical DNA binding sites, several observations support an overlap in function between homologous transcription factors encoded on accessory and core genomes. aTF7- and cTF7-overexpressing strains show a similar growth retardation and reduction of virulence, and aTf1 and cTf1 both are potent inducers of effector gene expression. In addition, a deletion mutant of cTF1 is less virulent, but can still colonize the plant [45,60]. The partial loss of virulence could be a result of redundancy between cTF1 and aTF1. Deletion of either of two of the shorter aTF1 homologs (FOXG_16414 and FOXG_17123) does not affect pathogenicity [45], but recently silencing affecting both aTF1 and cTF1 was shown to reduce virulence in both f. sp. lycopersici and f. sp. phaseoli [60]. Also host-induced gene silencing of aTF1, potentially silencing the entire gene family, has been reported in F. oxysporum f. sp. cubense on banana, which led to complete absence of disease symptoms [61].
If there indeed is such a large functional overlap between core and accessory homologs, then why does Fol contain transcription factor genes on the pathogenicity chromosome at all? And why—in some cases–even multiple homologs? One possibility is that the presence of the transcription factor (and other) genes on accessory chromosomes did not result from selective pressure on a particular functional advantage but is simply provoked by certain features of accessory chromosomes, such as high transposable element density or chromatin structure. A different, but not mutually exclusive, view is a selective advantage of gene duplications because of a dosage effect. In F. oxysporum f. sp. phaseoli, a correlation between virulence and the number of aTF1 homologs has been reported [35].
It should be noted, however, that although very similar, functions of core and accessory-encoded homologous transcription factors may not be completely redundant. For example, overexpression of aTF1 (FOXG_17458) induces expression of a transcription factor required for pathogenicity, whereas overexpression of cTF1 does not. Also, the short aTF1 (FOXG_17084) homolog can bind DNA with the same specificity, but is far less efficient in inducing SIX1 expression. At present, it cannot be excluded that the short homolog might even function as a negative regulator.
Is the transcriptional regulation of the pathogenicity chromosome relevant for horizontal transfer?
It is currently unclear over how large a phylogenetic distance the pathogenicity chromosome can be transferred. Up to now, transfer as only been experimentally demonstrated within the F. oxysporum species complex [7]. In such a case the cTF1 and SGE1 homolog in the recipient strain will be nearly identical to the chromosome donor strain, only accessory-encoded aTf1 homologs on regions other than the pathogenicity chromosome will be absent or different in the recipient. Interesting in this respect is a previous observation: when the pathogenicity chromosome is transferred to the non-pathogenic strain Fo-47, this strain gains pathogenicity, but is not very aggressive. Virulence is higher in those strains that gained not only the pathogenicity chromosome, but also a second small chromosome, corresponding to the duplicated region of chromosome 3/6 in the reference strain Fol4287 [7]. This region contains few if any effector genes, but many transcription factor genes, including several homologs of aTF1, aTF4, aTF6, aTF7, aTF8 (EBR2) and aTF9.
Genome analyses also suggest at least one ancient transfer event from a Fusarium species at a phylogenetic distance from Fol somewhere between F. verticillioides and F. graminearum [7]. If such a transfer were to occur again, the cTF1 homolog in the recipient would still be very similar to the cTF1 homolog in the donor strain, at least more similar than cTF1 is to aTF1. No additional accessory aTF1 homologs would be present in the recipient. We speculate that the transcription factors on accessory chromosomes do contribute to virulence, but many of their roles could be also partially fulfilled by their core-encoded homologs within the genus Fusarium.
For SGE1 homologs, significant functional differences have been reported between closely related species, like different Fusarium spp, but also between C. albicans and S. cerevisiae [23–25,33]. As described above, the DNA binding domain at the N-terminus is very conserved, and so is–as far as it has been tested–the DNA binding site [33], whereas the C-terminal domain is very variable [24,25]. For the yeasts C. albicans and S. cerevisiae, differences in target genes are caused by differences in the presence or absence of cis-acting promoter elements [33]. For F. graminearum and Fol, however, differences in target genes are caused by differences in the C-terminal domain, and transcomplementation can partially restore pathogenicity only in some cases [24]. Also in F. fujikuroi and F. verticillioides overexpression of their respective SGE1 homologs regulates a different set of genes [23,25].
Since the differences between the Sge1 homologs between different Fusarium species are so substantial that Sge1-mediated transcription regulation is extensively rewired, this may be a limiting factor to expression of effector genes from the Fol pathogenicity chromosome in another Fusarium species. Interestingly, transcomplementation of the Δsge1 mutant of Fol with CfWOR1 from C. fulvum resulted in restoration of effector gene expression but not in restoration of pathogenicity [28].
SGE1 overexpressors and the Δsge1 mutant enable identification of different potential target genes.
Previously, Jonkers and co-workers have looked at differentially expressed genes in a Fol SGE1 deletion mutant compared to WT using a microarray [24]. This set (1213 genes) is very different from the differentially expressed genes in the SGE1 overexpressors that we found (168 genes); only 15 genes are present in both sets. This shows that the different approaches are complementary for identification of target genes. The genes differentially expressed between WT and deletion strain are predominantly present on the core genome. Also, the Sge1 DNA binding site is not significantly enriched among up- or down-regulated genes in the deletion mutant (104 [out of 394] down-regulated genes with a Sge1 DNA binding site and 171 [out of 820] up-regulated genes, on a total of 4870 [out of 20935] genes with a Sge1 DNA binding site), in contrast to the SGE1 overexpressor. This may be because several transcription factor genes are differentially expressed in the deletion mutant, potentially regulating secondary targets. Also, the comparison of WT and SGE1 deletion mutant was of necessity conducted under axenic growth conditions which excludes finding targets that are not or very weakly expressed under those conditions, including most genes on the accessory genome.
What is the role of the other transcription factors encoded on the pathogenicity chromosome?
We have shown that aTf1 can activate effector gene expression. However, many more transcription factors are encoded on the pathogenicity chromosome. It is tempting to speculate that these transcription factors may control expression of some of the other plant-responsive genes. The core-encoded homologs of some of the other accessory transcription factors have been implicated in pathogenicity. cTF8 (EBR1) is required for full pathogenicity of Fol and F. graminearum, and orthologs of cTF4 and cTF9 are required for pathogenicity of F. graminearum (FGSG_10057, FGSG_10517 and FGSG_06651 respectively) [37,62]. Of these three transcription factors, we have only obtained a DNA binding site for cTf4, which is, like the DNA binding sites of aTf2, aTf5 and aTf7, not enriched among genes up- or down-regulated during infection. It is of course possible that there is not always a significant correlation between the presence of the DNA binding site and changes in expression during infection. This may occur when loss of pathogenicity is an indirect effect (via a second, downstream transcription factor), when only a particular combination of transcription factors regulates plant responsive genes, or when too many apparent binding sites are not functional, precluding detection of a significant association between binding site and gene regulation.
Of two transcription factors (cTf4 and aTf5), DNA binding sites are enriched on the accessory chromosomes, suggesting they may also act on genes of the accessory chromosomes. In F. graminearum, global gene regulatory network modelling revealed that species-specific genes are most often controlled by species-specific regulators, whereas genes conserved between species are controlled by conserved regulators [63]. Possibly, such a compartmentalized network structure of gene regulation may also apply to F. oxysporum.
Other putative functions of the transcription factors encoded on the pathogenicity chromosome could be downregulation of effector gene expression and returning to or maintaining a repressed, saprotrophic state. They may also be promoting horizontal transfer. It would be interesting to see if the pathogenicity chromosome can be lost, and if so, in which ways this may affect the phenotype of Fol and expression of core genes.
Materials and Methods
Cloning
In order to determine the DNA binding sites of the different transcription factors, the ORFs were cloned from cDNA. For this, RNA was isolated from axenic cultures and from susceptible infected tomato plants, between 1–2 weeks after inoculation. cDNA was generated as described below. For some transcription factors alternative start codons were tried, and the longest obtained PCR product was selected for cloning. For many transcription factors, 35 PCR cycles was insufficient to amplify clonable amounts of DNA, therefore, a re-amplification was done. Primers were designed in such a way that the subsequent product could be cloned with AscI, BamHI or SbfI, in frame with a N-terminal GST-tag in an Escherichia coli T7 expression vector pTH6838. The cloned ORF was checked by sequencing.
To express the transcription factor genes in Fol, the binary vector pRW2h was modified, yielding a plasmid with a right border (facilitating Agrobacterium tumefaciens mediated transformation), the FEM1 promoter, a multiple cloning site including XbaI, AscI, StuI, SbfI, BglII and ApaI, followed by the SIX1 terminator, the HPH resistance cassette and the left border. The transcription factor ORF was then cut out of pTH6838 with the same enzymes used to clone it in, and cloned into pRW2h_Pfem_MCS_Tsix1, again with the same enzymes. For those transcription factors of which the gDNA ORF was cloned, a PCR was done on Fol007 gDNA, isolated as described in [64] using the same primers initially designed for cloning of the cDNA. The PCR product was digested and cloned directly into pRW2h_Pfem_MCS_Tsix1. The cloned transcription factor ORFs were checked by sequencing.
Fol transformation
Fol was transformed via Agrobacterium mediated transformation, as described previously [65]. Transformants were monospored by pipetting 10–20 μl of sterile water on the emerging colony, and spreading this on a fresh PDA plate supplemented with cefotaxime and Hygromycine. After two days of growth at 25 degree Celsius, single colonies were picked and transferred to fresh plates. From these plates glycerol stocks were made and these are the transformants we worked with. Transformants were only selected on antibiotic resistance, and should contain one (or in rare cases more than one) random ectopic insertion of the T-DNA construct [26].
Microconidia production, harvest of mycelium, harvest of infected plants
Cultures for RNA isolation were grown as described below, except for experiments described in S8A Fig and Fig 9. These RNA isolations were done as described under ‘Deletion of SGE1 in aTF1 overexpressor background’ later in this section. For all other cases a small cube with mycelium from a PDA plate was used to inoculate a preculture of 50 ml liquid minimal medium (1%KNO3, 3% sucrose 0.17% YNB w/o amino acids or NH4) and grown for 3 to 5 days at 25°C, shaking 150–175 rpm. From this preculture microconidia were isolated by filtering the culture over miracloth and pelleting the microconidia in the filtrate at 2000 rpm for 10–15 min.
To harvest mycelium for RNAseq analysis of transcription factor overexpressors or for quantification of transcription factor transcripts by Q-RT-PCR, microconidia were suspended in a small volume of minimal medium, counted, and used to inoculate 100 ml liquid minimal medium with 2.5 * 10^8 microconidia. This culture was grown for 2 days at 25°C, shaking 150–175 rpm before mycelium was harvested by filtering the culture over a double layer of miracloth. The mycelium in the filter was washed once with 50–100 ml sterile water, scraped from the miracloth and snap frozen in liquid nitrogen. Of each condition, three independent biological replicates were sampled.
To harvest material for RNAseq analysis of infected plants, Fol4287-infected tomato roots were harvested nine days post inoculation. Infections were performed as described below (for the bioassays). Fol4287 mycelium from axenic cultures was harvested from five day old cultures inoculated from plate in 100 ml liquid minimal medium (1%KNO3, 3% sucrose 0.17% YNB w/o amino acids or NH4), 25°C, 150–175 rpm.
RNA isolation, cDNA synthesis & RNAseq
RNA was isolated as described earlier, using a Trizol extraction on mycelium ground in liquid nitrogen, followed by DNase treatment and purification over RNeasy RNA purification columns, according to the instructions of the manufacturer (Qiagen).
Synthesis of cDNA was performed using 1 μg of RNA, poly dT primers, Promega RNasin (ribonuclease inhibitor) and Gibco Superscript II RNase H− Reverse transcriptase, according to instructions of Gibco.
Of 2 μg of total RNA of each biological replicate, polyadenylated RNA was amplified and ligated to adapters to make a library suitable for multiplex illumina paired-end sequencing. Each sample was barcoded and sequenced in 8 different lanes. After de-multiplexing, total reads of the different lanes were combined. This rendered one file of reads per biological replicate.
Quantitative RT-PCR
Quantitative PCR was performed with a model 7500 Real Time PCR system (Applied Biosystems) and Solis BioDyne 5x HOT FIREPol Eva Green qPCR Mix Plus (ROX). Primers used for Q-RT-PCR where designed to amplify fragments of approximately 100 bp and tested for primer efficiency and melting curve (S1 Data). 1 μL of cDNA was used per sample, two technical replicates were performed for each sample. Transcription elongation factor 1α (EF1α) gene expression was used as a reference, and RNA that was not transcribed into cDNA as a gDNA contamination control. The following formula was used to calculate the amount of DNA: [DNA] = (1/E^ Ct_sample) -(1/E^Ct_control), with E = primer efficiency, Ct_sample = Ct value of the test sample, using WT or TF overexpressor cDNA as a template and Ct_control = Ct value of no cDNA control sample (to check for gDNA contamination), with the same primer pair. The comparison with EF1α was made as follows: DNA_TF/DNA_EF1α. Standard deviations of the two technical replicates per sample were calculated with the following formula: Standard deviation = √((stdev DNA_TF /average DNA_TF)^2 + (stdev DNA_EF1α /average DNA_EF1α)^2))* (DNA_TF /DNA_EF1 α).
Whole genome read mapping
The Illumina reads (125 bp paired end, insert size around 200–500 bp) were mapped to the annotated genome of Fol4287 (Fusarium Comparative Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/), annotation 3) using CLC Genomics Workbench version 6.5.1 module (CLC bio, Aarhus, Denmark). Reads were imported as: illumina (pipeline 1.8 and later), paired-end reads, insert size 100–600 bp, remove failed reads. Imported reads were trimmed to remove any remaining adapter sequences or low quality reads. Quality scores and ambiguous nucleotides were trimmed with standard settings (limit 0.05, ambiguities 2). Adapters were trimmed by checking for the presence of the Truseq Universal adapter (minus strand) and the presence of the Truseq index adapter (plus strand) with the following parameters: mismatch = 2, gapcost = 3, cutoff ns, cutoff at end 6, action: remove adapter.
The following gene models were manually added to the annotation:
Supercontig_2.36 135864–136180 minus strand FOXG_SIX14; Supercontig_2.51 62412–62758 minus strand FOXG_SIX12; Supercontig_2.51 65216–65878 plus strand FOXG_SIX7; Supercontig_2.22 806692–807024 minus strand FOXG_SIX11; Supercontig_2.22 44647–44970 minus strand FOXG_PEG4 (Putative Effector Gene 4); Supercontig_2.36 468654–469317 minus strand FOXG_SIX8-36; Supercontig_2.51 6999–7662 minus strand FOXG_SIX8-51.
Reads were mapped to the annotated genome with parameters: Organism type = eukaryote. Exon discovery according to standard settings: relative expression level = min 0.2, min reads = 10, min length = 50 bp. Additional downstream bases = 0. Additional upstream bases = 0. Minimum length fraction = 0.9. Minimum similarity fraction = 0.95. Minimum number of reads = 10. Map only intact pairs, count paired reads as one. Unspecific match limit = 10. Expression value = Total number of mapped reads and reads per kb per million mapped reads (RPKM).
Whole genome differentially expressed gene calling
Differentially expressed genes were called for pairwise comparisons of the total number of mapped reads (three replicates), using the Bioconductor DEseq software [52]. After normalization for library size (estimateSizeFactors) and variance estimation (estimateDispersion) with parameters “method = blind, sharingMode = fit-only”, Nbinominal testing and the Benjamin-Hochberg multiple testing adjustment procedure were used [52]. For the transcription factor gene overexpressing transformants (all compared pairwise to the Fol007 Psix1GFP samples), all genes that had an adjusted p value <0.1 for both overexpressors were considered significant. For the samples from infected plants (compared to the Fol 4287 control samples) all genes with an adjusted p value < 0.05 were considered significant. The output file gives the mean normalized mapped reads per sample (mean of the three replicates), pvalue and adjusted pvalue.
Analysis of differentially expressed genes
For all pairwise comparisons the normalized total mapped reads were collected, and every count of zero reads was replaced by 0.1 (0.1 is roughly half of the lowest number of normalized total mapped reads in all comparisons, this allows calculation of -an approximate- fold change for each gene). Fold change and log2 fold change were calculated. To visualize expression differences in a heatmap, all genes considered differentially expressed in one of the comparisons were listed and the log2 fold change for each condition was listed in seven subsequent columns. In this list, each value not reaching the significance threshold was replaced by ‘0’ (indicating no fold change). This list was separated into three lists based on subgenome and each of these lists were clustered on gene and condition in Gene Cluster 3.0, uncentered correlation, average linkage. Results were visualized in Java Treeview.
Next to this, the differentially expressed genes were subdivided in lists of up-regulated and down-regulated genes. These lists were used to count the contributions of different subgroups to each category (for example: number of aTF1 up-regulated genes that is located on the pathogenicity chromosome). Hypergeometrical distribution tests were used to determine significant enrichments or depletions among different categories. The adjusted p value was reached by multiplying the p value with the total number of tests performed.
To determine which genes have a MIMP in their promoter, two kb upstream the ATG of each gene was searched for the presence of complete (both inverted repeats present) or partial MIMPs. Any missing SIX gene, of which a MIMP had been demonstrated in the promoter earlier [34], was manually added to the list.
Read mapping and differentially expressed gene calling for transposable elements
The Illumina reads (125 bp paired end, insert size around 200–500 bp) were mapped to the fasta file with all repetitive/transposable elements as described above, except the following parameters: Organism type = prokaryote, unspecific match limit = 30. Reads were normalized as the number of reads per 20*10^6 uniquely mapped reads to the total genome for that particular sample.
For the more detailed analysis the same pairwise comparisons were made as described above. Every count of zero reads was replaced by 0.1, the data was log10 transformed and a T-test was performed on the log10 transformed normalized total mapped reads. All sequences that compared differently (p<0.05) were counted.
Bioassay & growth assay
Bioassays were performed as described earlier [66]. Briefly, tomato seedlings of 10 to 11 days were trimmed at the main root and dipped in a Fol microconidia suspension of 0.5*10^7 microconidia/ml for at least 1 minute. The seedlings were potted in soil in individual pots and grown in the greenhouse at 25°C for three weeks. At the time of harvest, the above ground parts were cut off at the cotelydons and scored for fresh weight and disease index. The disease index ranges from 0 (no symptoms), 1 (thickening of hypocotyl, formation of lateral roots), 2 (one brown vessel), 3 (up to ¾ of the vessels show browning, asymmetric development) to 4 (all vessels brown, severe growth retardation, death).
Growth assays were performed by positioning a droplet of spores on the middle of a PDA or CDA plate, growing the fungus at 25°C for 5 days, and measuring the colony diameter.
GFP assay
GFP fluorescence was measured on a Fluostar optima platereader (BMG Labtech). For this dilutions of 10^8, 10^7, 10^6 and 10^5 microconidia per ml were made and 200 μl of each suspension was pipetted in a sterile, flat bottom, black 96 well plate (Greiner). The plate was shortly mixed and measured from the bottom, with 470–10 nm excitation and a 510–10 nm emission filter. The plates were kept o/n at 25°C, and measured again the next day, same settings. No differences were observed between the days (apart from a slight increase in fluorescence due to growth).
DNA-binding arrays
Details of the design and use of PBMs have been described elsewhere [38,67–69]. Here, we used two different universal PBM array designs, designated 'ME' and 'HK', after the initials of their designers, as described in [70]. Briefly, we used 150 ng of plasmid DNA in a 15 μl in vitro transcription and/or translation reaction using a PURExpress In Vitro Protein Synthesis Kit (New England BioLabs) supplemented with RNase inhibitor and 50 μM zinc acetate. After a 2-h incubation at 37°C, 15 μl of the mix was added to 155 μl of protein-binding solution for a final mix of PBS/2% skim milk/0.2 mg per ml BSA/50 μM zinc acetate/0.1% Tween-20. This mixture was added to an array previously blocked with PBS/2% skim milk and washed once with PBS/0.1% Tween-20 and once with PBS/0.01% Triton-X 100. After a 1-h incubation at room temperature, the array was washed once with PBS/0.5% Tween-20/50 mM zinc acetate and once with PBS/0.01% Triton-X 100/50 mM zinc acetate. Cy5-labeled anti-GST antibody was added, diluted in PBS/2% skim milk/50 mM zinc acetate. After a 1-h incubation at room temperature, the array was washed three times with PBS/0.05% Tween-20/50 mM zinc acetate and once with PBS/50 mM zinc acetate. The array was then imaged using an Agilent microarray scanner at 2 μm resolution. Images were scanned at two power settings: 100% photomultiplier tube (PMT) voltage (high), and 10% PMT (low). The two resulting grid images were then manually examined, and the scan with the fewest number of saturated spots was used. Image spot intensities were quantified using ImaGene software (BioDiscovery).
Calculation of spot intensities was done as described in [38]. In summary, bad spots (spots that had scratches, dust flecks or other imperfections) were flagged manually and removed from subsequent analysis. The PBM signal intensity at each spot was normalized by the corresponding amount of dsDNA. To correct for any possible nonuniformities in hybridization, these normalized PBM intensities were then adjusted according to their positions on the microarray. Each spot was considered to be at the center of a block of spots [70]. The difference between the median normalized intensity of the spots within the block and the median normalized intensity of all spots on the microarray was subtracted from the normalized intensity at that particular spot.
Calculation of 8-mer Z- and E-scores was performed as previously described [38,71]. Z-scores are derived by taking the average spot intensity for each probe containing the 8-mer, then subtracting the median value for each 8-mer, and dividing by the standard deviation, thus yielding a distribution with a median of zero and a standard deviation of one. E-scores are a modified version of the AUROC statistic, which consider the relative ranking of probes containing a given 8-mer, and range from −0.5 to +0.5, with E > 0.45 taken as highly statistically significant [67].
DNA-binding site inference
DNA binding sites were determined as described in [72]. The oligo-binding array returns for each transcription factor a set of eightmers and corresponding E-scores that indicate the likelihood that the protein binds this eightmer. This set of eightmers contains both the forward and reverse binding eightmers and may represent different binding motifs for a single transcription factor. Hence to infer binding motifs for a transcription factor from a set of eightmers, we need to first cluster eightmers into similar groups, where we expect at least two clusters (‘forward’ and ‘reverse’) for each transcription factor, unless the binding motif is a palindrome. First we remove unreliable eightmers (those that have a score < 0.45). We then perform pairwise Smith-Waterman alignments using the water program from the EMBOSS package (with options: -nobrief -gapopen 5.0 -gapextend 2.0) to obtain a sequence similarity measure (the Smith-Waterman score) for each eightmer-pair. We take 40.0 –the Smith-Waterman score as a pairwise distance and use hierarchical clustering as implemented in scipy (average linkage: UPGMA) to obtain a hierarchical clustering of the eightmers. We split the resulting clustering trees into two clusters and manually checked whether these correspond to ‘forward’ and ‘reverse’ strands. We find that this is the case for all transcription factors except FOXG_15625 that probably has a palindromic binding site and FOXG_04904 for which we did not find ‘reverse’ strand eightmers. In the cases where we could identify ‘forward’ and ‘reverse’ strands we added the reverse complement of ‘forward’ strand sequences to ‘reverse’ strand sequences, in other cases we simply merged both clusters as they were. We aligned the sequences and obtained a sequence logo (as shown in Fig 3A) for these alignments with WebLogo [73].
DNA binding site enrichment analysis
The following sequence motifs were used to search the regions 1000 bp upstream or 1000bp downstream of the annotated transcriptional start site (Fusarium Comparative Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/), annotation 3); aTF2: CAAAC, cTF4: AGCC[A,G,C,T]TA, aTF5: CACGT, aTF7: G[A,G,C]GGCT, aTF1:T[A,G]CCG, SGE1: TTA[A,G][A,G][G,C]TT, effector motif: AACTGCCGA. For the upstream regions we used fasta files with promoter regions that we downloaded from the Broad Institute. We used custom Python scripts to append promoter regions for SIX genes that were not part of the annotation, based on the reported locations in [34]. We used custom Python scripts to make a fasta file with sequences that correspond to the first 1000 bp downstream from the first ATG based on the transcript gtf-file downloaded from the Broad Institute or–for SIX genes that were not part of the annotation–based on locations reported in [34]. We counted the number of genes with one or more motifs in the upstream regions, forward and reverse orientation separately. We did the same for two or more motifs and three or more motifs. Significant enrichment of genes with binding sites in the upstream regions in certain subgroups (accessory genes, plant-induced genes, etc.) was tested with a Hypergeometric test, with a P value < 0.01 after Bonferroni correction.
Phylogenetic trees
To define TF families we used blastp to search for homologs in 12 Fusarium oxysporum species (Fusarium Comparative Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/)[74]. We only included hits that have an E-value < 1e-5 and for which the alignment returned by BLAST spans more than 60% of both the query and the subject sequence. We used a custom Python script to cluster all hits into families using single linkage clustering and used Clustal Omega to construct multiple sequence alignments per family [75]. We trimmed the alignments using trimAl (-strictplus) [76], inspected and manually curated them. We used PhyML (with options: -q -b -2 -v e -a e) to infer phylogenies [77]. For large families we pruned the tree such that we keep the last common ancestor of the TFs that lie on chromosome 14 and their nearest neighbour that lies on the core, as root of the pruned tree.
For each of the seven transcription factor families tested for DNA binding, we searched for the occurrence of conserved domains from the Pfam database (version 27.0) using hmmscan from the hmmer package. We manually checked for presence of residues that are conserved in the Pfam seed alignment of the DNA binding domain in the multiple sequence alignments (S3 Fig).
Transposable/repetitive element list
We compiled a list of sequences corresponding to putative transposable elements by extracting DNA sequences for elements identified in a thorough analysis of chromosome 14 [34]. We combined these elements by elements identified by running RepeatMasker (with RepBase19.11) and extracting all sequences that were not denoted as a low-complexity region or simple repeat. We filtered out multiple occurrences of identical sequences.
LacZ activation assay
For the transcription activation assay in yeast, plasmids (Ptef1-expression vector [B3909] with and without WOR1, Wor1 DNA binding site–WT, mutated or empty–in front of UAS-less CYC1 promoter followed by the LacZ reporter gene [B3946]) and strains (S. cerevisiae Sigma 2000 ΔYEL007 /ΔYHR177) were a kind gift from Alexander D. Johnson and are described in [42]. To express SGE1 in yeast, the SGE1 ORF was amplified from gDNA and cloned behind the TEF1 promoter in plasmid B3909 using SpeI and XhoI restriction enzymes. To express aTF1 or cTF1 in yeast, for each gene both exons were amplified from gDNA and fused together in an overlap PCR, to create an intronless sequence. This sequence was then cloned behind the TEF1 promoter in plasmid B3909 using SpeI and SalI restriction enzymes. To clone the Tf1 DNA binding site (WT or mutated), oligo pairs were ordered corresponding to part of the SIX1 promoter (-335 to –290 relative to the ATG) containing two Tf1 motifs flanked by 5 bp on each end plus sticky ends corresponding to the XhoI restriction site. Oligos were phosphorylated using T4 PNK (Fermentas), ligated into XhoI-digested and phosphatase treated B3946 plasmid. All plasmids were sequenced to verify the insert sequence and orientation. Oligos are listed in S1 Data, tab: ‘primers’.
Plasmids containing WOR1, SGE1, aTF1 or cTF1 and LacZ reporter plasmids were co-transformed to yeast strain Sigma 2000 ΔYEL007 /ΔYHR177 according to [78]. LacZ activity was assayed as follows. Transformed yeast cells were grown in SD medium lacking Uracil and Histidine. Before cells were harvested, OD600 was measured and cells were pelleted. The cell pellet was resuspended in 150μl Z-buffer with β-mercaptoethanol (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1mM MgSO4, 1 mM β-mercaptoethanol, pH 7), 50 μl chloroform and 20 μl 0.1% SDS were added, tubes were vortexed for 15 seconds and 700 μl pre-warmed (30°C) Z-buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1mM MgSO4, pH 7) with ONPG (1 mg/ml) was added at t = 0. Tubes were incubated at 30°C until the reaction started to turn yellow. The reaction was stopped with 500 μl 1M Na2CO3 and the time recorded, Tubes were centrifuged 2 min. at 14000 rpm and the OD of the supernatant was measured at 420 nm. Miller units were calculated as follows: (A420*1000)/(A600*minutes*ml culture).
Deletion of SGE1 in aTF1 overexpressor background
To make aTF1 overexpressors in the Fol4287 wild type background, the hygromycin resistance cassette (HPH) in the plasmid described above (Pfem1aTF1-HPH cassette) was exchanged for a phleomycine resistance cassette (BLE), using XbaI plus PacI (insert) and SpeI plus PacI (vector) restriction enzymes. The resulting plasmid (Pfem1aTF1-BLE cassette) was transformed to Fol4287 and transformants were selected on zeocine. An empty plasmid (pRW1p: containing only the BLE cassette [79]) was transformed to Fol4287 in parallel, as a negative control. Ten independent zeocine resistant colonies of each transformation were monospored and checked for SIX1 and SIX3 expression. For this, liquid cultures (100 ml 1% KNO3, 3% sucrose, 0.17% YNB w/o NH4 and aa.) were inoculated from plate and mycelium was harvested after 5 days at 25°C, 150–175 rpm. RNA isolation, cDNA synthesis and Q-RT-PCR were performed as described above. SIX1 and SIX3 levels were normalized to EF1-α. Two out of ten aTF1 transformants showed induction of SIX1 and SIX3 expression. One of those was selected for deletion of SGE1.
Deletion of SGE1 in the selected Fol4287 aTF1 overexpressor was done as described in [26], using the same deletion construct and the same PCR control for deletion of SGE1. To check transformants (ectopic and in locus) for SIX1 and SIX3 expression, liquid cultures (100 ml 1% KNO3, 3% sucrose, 0.17% YNB w/o NH4 and aa.) were inoculated from plate and mycelium was harvested after 5 days at 25°C, 150–175 rpm. RNA isolation, cDNA synthesis and Q-RT-PCR were performed as described above. SIX1, SIX3, SGE1 and aTF1 levels were normalized to EF1-α. Primers used are listed in S1 Data, tab: ‘primers’.
Supporting Information
Phylogenetic trees of the protein sequences of the different transcription factor families, including homologs in Fol, other F. oxysporum ff. spp. and other Fusarium species. A) Family of aTf4 (FOXG_14201), a C2H2 zinc finger transcription factor—this transcription factor has no additional accessory homologs in Fol. B) aTf3 (FOXG_17266), a C2H2 zinc finger transcription factor with no homologs on the core genome, and only two homologs in accessory regions in other formae speciales. C) Family of aTf6 (FOXG_14211), a C2H2 zinc finger transcription factor—this transcription factor has no additional accessory homologs in Fol. D) Family of aTf7 (FOXG_14275), a C2H2 zinc finger transcription factor. This transcription factor has seven additional accessory homologs in Fol4287. E) Protein family of aTf1 (Ftf1; FOXG_14257, FOXG_17458, FOXG_16414) of Zn(2)Cys(6) zinc finger transcription factors. This transcription factor has seven additional accessory homologs in Fol4287. F) Family of aTf8 (FOXG_14277), a Zn(2)Cys(6) zinc finger transcription factor. This transcription factor has five additional accessory homologs in Fol4287. G) Family of aTf9 (FOXG_14274, FOXG_14137, FOXG_14220) of bZIP leucine zipper transcription factors. This family has seven additional accessory homologs in Fol4287. H) Family of aTf2 (FOXG_17260), a forkhead transcription factor with one homolog on the core genome and one homolog on the accessory chromosomes. Only three other forkhead transcription factor genes are present in the Fol4287 genome. I) Family of aTf5 (FOXG_14230), a bZIP leucine zipper transcription factor—this transcription factor has no additional accessory homologs in Fol.
(PDF)
In-frame potential startcodons (ATG) are indicated with red arrows and are bold and underlined, other ATG codons are bold. Red bases indicate single basepair indels. The following genes have a ‘long’ predicted ORF, according to the Broad annotation (the number refers to the FOXG number of the Broad annotation): 09390, cTF1, core genome; 17458, pathogenicity chromosome, used for overexpression & RNAseq; 14257, pathogenicity chromosome, 14422, accessory chromosome 15; 15059, accessory region on chromosome 1. The remaining genes have a shorter predicted ORF according to the Broad annotation, namely: 16414, pathogenicity chromosome; 17123, accessory chromosome 6, 17084, accessory chromosome 3/6, used for overexpression; 14000, accessory chromosome 3/6; 12589, accessory chromosome 3/6, 12539, accessory chromosome 3/6. The genes with a long predicted ORF start at the ATG indicated with red arrow number 1. All remaining genes have a 17 base pair deletion (compared to the core homolog cTF1/>09390), removing this ATG. Their predicted startcodon is the next in-frame ATG (red arrow 2). In addition, all homologs with the 17 bp deletion also have a more upstream stop codon– 300 bp upstream of the stopcodon in cTF1. This means that all ORFs starting at the position indicated by red arrow 2, start 117 bp later and stop 300 bp earlier than the long TF1 homologs, rendering ORFs of around 2800 bp. These genes we refer to as having a short ORF. The ORFs starting at the position indicated by red arrow 1 are around 3200 bp. These genes we refer to as having a long ORF.
(PDF)
For each family we predicted domain architecture for its members. We here show for each family, from the multiple sequence alignment used to generate its phylogeny (S1 Fig), those regions that correspond to a DNA binding domain. The background color of a residue corresponds to its conservation in the alignment, where darker colors indicate more conservation. Residues that are conserved in the seed alignment from Pfam and are present in at least two sequence are indicated in bold on top of the alignment. Residues that are conserved in the Pfam seed alignment but are not present in at least two sequences are in normal font. Gene names correspond to names and species or ff. spp. mentioned in Fig 2and S1 Fig. Core-encoded transcription factors of F. oxysporum (Fol4287), F. verticillioides, F. graminearum and F. solani are indicated in red. Accessory transcription factors from Fol tested for DNA binding specificity are indicated in bold.
(PDF)
A) The number of genes with one or more transcription factor DNA binding site (TF DBS) in the 1000 bp upstream of the predicted transcriptional start site, for the complete genome, and for a subset of genes (up- or down-regulated during infection). Boxes indicate a significant enrichment (p value <0.01 after Bonferroni correction). The motif found in effector gene promoters earlier (aacTGCCGa and overlapping with the Tf1 DNA binding site has been included. For all categories the total number of genes is given (ALL, green), the number of core genes (CORE, blue) and the number of accessory genes (purple). Accessory genes are subdivided in total (ACC), accessory genes not on the pathogenicity chromosome (no14) and the accessory genes on the pathogenicity chromosome (only_14). B) As A, but for the 1000 bp downstream the ATG. C) As A, but only for the Tf1 DNA binding site (≥3 occurrences), the motif found in the promoters of effector genes, overlapping with the Tf1 DNA binding site (≥1 occurrences) and the Sge1 DNA binding site (≥1 occurrences). The following categories are additional: genes UP or DOWN regulated in the aTF1, cTF1 or SGE1 overexpressors, genes for small secreted proteins, genes with a MIMP in their promoter, genes coding for proteins found in xylem sap of infected plants. D) As C, but for the 1000 bp downstream the ATG.
(PDF)
A) Estimated percentage, by visual inspection, of GFP-fluorescent spores in independent transformants of different transcription factor overexpression constructs. x: this transformant does not exist (misnumbered), *: of the background strain (Psix1GFP—WT) the same transformant was tested multiple times, each number represents therefore a replicate instead of a different transformant. B) Relative expression of different transcription factor genes in WT and their respective overexpressors as determined by Q-RT-PCR. Relative expression is calculated as TF expression/expression of EF1α. Minimally three independent transformants were tested per transcription factor. Error bars indicate the standard deviation of two technical replicates. C) Growth rate of the different transcription factor overexpressors. Minimally three independent transformants were tested per transcription factor, on both rich medium (potato dextrose agar, PDA, top panel) and complete minimal medium (Czapek Dox Agar, CDA, bottom panel). Stars indicate significant differences compared to the background strain (T-test, p<0.01). Error bars indicate the standard deviation of minimally two independent replicates. D) Bioassay on susceptible tomato plants. Minimally three independent transformants were tested per transcription factor. Control treatments were: mock, WT (this is the WT used to make the background strain, Fol007), Psix1GFP (the background strain, WT + reporter construct Psix1GFP), Psix1GFP + RFP (the background strain plus a constitutively expressed RFP construct). Top panel: average plant weight (gram) 21 dpi. Error bars indicate standard deviation. Stars indicate a significant difference from the background strain (Psix1GFP, T-test, p<0.01). Bottom panel: disease index (0–4 arbitrary units) 21 dpi.
(PDF)
A) Top three Venn diagrams show the overlap of sets of differentially expressed genes between two independent transformants of each transcription factor. The overlapping genes are depicted in red and these are the genes considered for all further analyses. Lower six Venn diagrams: overlap between target genes in the different overexpressors, up-regulated (left three diagrams) or down-regulated (right three diagrams). B&C) Number of up- or down-regulated genes in different subcategories. Significant enrichment or depletion in each subcategory was tested with a hypergeometric test, P value < 0.01 after Bonferroni correction. Orange cells: enrichment, blue cells: depletion. Columns: whole genome (all genes), planta UP (genes up-regulated during infection), aTF1 UP, cTF1 UP, SGE1 UP (genes up-regulated in the aTF1, cTF1 or SGE1 overexpressors, a1c1S1 UP (genes up-regulated in all three overexpressors), planta DOWN (genes down-regulated during infection), aTF1 DOWN, cTF1 DOWN, SGE1 DOWN (genes down-regulated in the aTF1, cTF1 or SGE1 overexpressors, a1c1S1 DOWN (genes down-regulated in all three overexpressors), MIMP (genes with a miniature impala transposable element (MIMP) in their promoter), XS proteins (genes of which the protein product was found in the xylem sap of infected tomato plants [34]). Rows: all (all genes), core (all genes located on the core genome), pathogenicity chr. (all genes on the pathogenicity chromosome), other accessory chr. (all accessory genes, except those on the pathogenicity chromosome), in planta UP all (all genes up-regulated during infection), UP core (genes located on core chromosomes and up-regulated during infection), UP pathogenicity chr. (genes located on the pathogenicity chromosome and up-regulated during infection), UP accessory chr. (genes on the accessory regions, except those on the pathogenicity chromosome, and up-regulated during infection), in planta DOWN (all genes down-regulated during infection), SIX genes (all SIX genes), XS proteins (genes of which the protein product was found in the xylem sap of infected tomato plants [34]), XS proteins w/o SIX proteins (genes coding for xylem sap proteins minus the SIX genes), mimp (genes with a MIMP in their promoter), mimp on accessory chr. (genes on the accessory regions with a MIMP in their promoter), mimp on pathogenicity chr. (genes on the pathogenicity chromosome with a MIMP in their promoter), mimp on core (genes on the core chromosomes with a MIMP in their promoter), mimp w/o SIX genes (genes with a MIMP in their promoter, without the SIX genes), mimp on path. chr. w/o SIX (genes on the pathogenicity chromosome with a MIMP in their promoter, without the SIX genes). D) Number of up- or down-regulated transposable element transcripts in different subcategories. All sequencing reads from the overexpressors and reads from infected plant material were mapped to a fasta file in which the sequence of each repetitive element, plus each previously annotated transposable element, is present once. The number of reads was normalized to the number of reads mapped uniquely to the whole genome for each sample. The number of reads mapping to each different sequence was counted and differentially expressed sequences were called. Significant enrichments or depletions were tested with a hypergeometric test, P value < 0.01 after Bonferroni correction. Orange cells: enrichment, blue cells: depletion. Columns: ALL (all genes or transposable elements), UP (up-regulated genes or transposable elements), DOWN (down-regulated genes or transposable elements), aTF1A & aTF1E (two independent aTF1 overexpressors), cTF1C & cTF1D (two independent cTF1 overexpressors), SGE15 &SGE1M (two independent SGE1 overexpressors), in planta (Fol sampled from infected plant material). Accessory; all genes located on the accessory regions, TE: transposable elements. Note that the reads mapping to a single sequence may be derived from many different locations on the genome, so each sequence represents ‘net’ expression. Because most transposable elements are located on accessory chromosomes, comparisons were made to those regions specifically. The portion of repetitive/transposon sequences down-regulated in the different transformants is very similar to the portion of accessory genes down-regulated. The number of transposons up-regulated in the different transformants is lower than the portion of genes up-regulated on the accessory chromosomes. Both may be due to inclusion of transposable elements in the large scale gene repression on the accessory chromosomes, given that we look at the net expression for each sequence. Also during infection—tested with a strain that does not show the large accessory gene repression—no increased up-regulation of transposon transcripts is observed.
(PDF)
Relative expression was determined by Q-RT-PCR, and calculated as TF expression /expression of EF1α. Two independent transformants were tested per transcription factor. Plants were infected with strain Fol007 and roots were harvested for RNA extraction 9 dpi. Error bars indicate the standard error of two (during infection) or three (all other samples) independent biological replicates.
(PDF)
A) Relative expression of aTF1, GFP and SIX3 compared to expression of the reference gene EF1α determined by Q-RT-PCR. Expression was measured in nine different independent aTF1 overexpressors (a, b, c, e, f, g, j, k and o) and in the background strain (Psix1GFP). aTF1 is expressed from the endogenous locus and–in the overexpressors–from an additional overexpression construct. GFP is expressed by the SIX1 promoter, from the original locus. SIX1 and SIX3 are representative effector genes. Relative expression is calculated as gene expression/expression of EF1α. Error bars indicate standard deviation. B) clustering of differentially expressed genes (Padjusted < 0.01) after expression of aTF1, cTF1 or SGE1 from the FEM1 promoter, by log2 fold change values for each differentially expressed gene. The log2 fold change values of genes that are not significantly different from the control are set to zero. Two independent transformants were studied per transcription factor, resulting in six independent conditions. The tree reflects the similarity between the transcriptional changes in each sample.
(PDF)
Heatmap of differentially expressed genes during infection or after in aTF1, cTF1 or SGE1 overexpressors, divided per subgenome (the pathogenicity chromosome, other accessory regions and core genome). Displayed is the log2 fold change for each differentially expressed gene (rows), with yellow indicating up-regulation compared to the control (hues cover the log2 fold range from 0 to 5). Blue indicates down-regulation compared to the control (hues cover the log2 fold range from 0 to -5). The log2 fold change values of genes that are not significantly different from the control are set to zero. Each condition is a separate column, with two independent transformants (thus 2 columns) per transcription factor. The order of the rows reflects clustering of similar expression patterns. The first seven columns are the same as in Fig 8A, the eighth column is additional (column header highlighted in red). Wild type strain Fol4287 was used for in planta samples, and for Fol4287 samples from flask (compared to each other in the first column). Fol007 is a wild type strain very similar to Fol4287 and was used to create the background strain (Fol007 + Psix1GFP reporter construct) for the overexpressors. Overexpressors and their background strain were compared in column two to seven. The eighth column compares the two wild type strains to each other (Fol4287 and Fol007+Psix1GFP). The red bar highlights the group of genes that is expressed lower in Fol 4287 WT and in the overexpressors in Fol 007 + Psix1GFP background, compared to Fol007 + Psix1GFP.
(PDF)
Expression (of the background strain, top row, green) or the difference in expression with the background strain (of the overexpressors, six bottom rows, different shades of red) in RPKM or ΔRPKM (log10 scale). On the x-axis, the genes of SC 22, SC 36 and SC 51 are plotted, in the same order as they appear on the supercontig (SC). Only positive numbers are shown for the overexpressors (ΔRPKM)–down-regulated genes are not visible. No distinction has been made between significant and non-significant up-regulation—all differences are shown. The second and third rows show two independent aTF1 overexpressors (dark red), the fourth and fifth rows show two independent cTF1 overexpressors (bright red), the sixth and seventh rows show two independent SGE1 overexpressors (orange). The dashed box indicates the region that is up-regulated in one cTF1 overexpressor and one SGE1 overexpressor.
(PDF)
All sequencing reads from the overexpressors and reads from infected plant material were mapped to a fasta file where the sequence of each repetitive element, plus all each previously annotated transposable element, is present once. The number of reads was normalized to the number of reads mapped uniquely to the whole genome for each sample. In the graph, the total number of reads assigned to transposable elements per 20 million reads uniquely mapped to the reference genome are shown. Of each transcription factor overexpressor (aTF1, cTF1 or SGE1) two independent transformants are shown. No consistent significant effects on overall transposon expression were observed in any of the overexpressors. However, the total amount of transposon-derived reads during infection was reduced.
(PDF)
Bioassay on susceptible tomato plants. Two independent transformants were tested for both ectopic and in locus integration of the SGE1 deletion construct in the aTF1 overexpressor (aTF1 OE ectopic and aTF1 Δsge1, respectively). Control treatments were: mock, Fol4287 (WT), Δsge1 (SGE1 deletion mutant in Fol4287), aTF1 OE (aTF1 overexpressor in Fol4287). Left panel: average plant weight (gram) 21 dpi. Error bars indicate standard deviation. Right panel: disease index (0–4 arbitrary units) 21 dpi.
(PDF)
This file contains an overview of the transcription factors mentioned in this study, an overview of the results of the DNA binding assay, an overview of DNA binding site occurrences, overviews of genes up- or down-regulated in aTF1, cTF1 and SGE1 overexpressors, an overview of genes with a MIMP within 2 kb upstream of the ATG, genes of which the protein product is detected in xylem sap and genes up- or down-regulated in planta, a list of the primers used in this study and the mapped read counts + RPKM values for all RNA-seq samples.
(XLSX)
Acknowledgments
The authors acknowledge Ernst-Jan Eggers for pioneering and contributing in generating SGE1 overexpressors. We thank Alexander Johnson and Matthew Lohse for kindly sharing plasmids and strains, and Peter van Dam for his generous assistance in managing the RNAseq data and performing the MIMP search.
More than grateful we are for the help of Petra M. Houterman, Wim de Leeuw, Huub Hoefsloot and Antje van der Does-Bianchi in the RNAseq experiment, for Mila C. Blekemolen, Nico Tintor, Marijn Knip, Ludek Tikovsky and Harrold Lemereis, for their help in performing the bioassays, for Gertien Smits and her laboratory, who have been very kind and helpful in assisting measurements with their GFP platereader and, finally, to Ben J.C. Cornelissen, Nico Tintor and Harrold A. van den Burg for helpful suggestions and critical reading of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Netherlands Organization for Scientific Research (nwo.nl) through Veni grant 016.121.067 to HCvdD. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Soyer JL, El Ghalid M, Glaser N, Ollivier B, Linglin J, et al. (2014) Epigenetic control of effector gene expression in the plant pathogenic fungus Leptosphaeria maculans. PLoS genetics 10: e1004227 10.1371/journal.pgen.1004227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, et al. (2015) Fungal effectors and plant susceptibility. Annu Rev Plant Biol 66: 513–545. 10.1146/annurev-arplant-043014-114623 [DOI] [PubMed] [Google Scholar]
- 3.Schotanus K, Soyer JL, Connolly LR, Grandaubert J, Happel P, et al. (2015) Histone modifications rather than the novel regional centromeres of Zymoseptoria tritici distinguish core and accessory chromosomes. Epigenetics Chromatin 8: 41 10.1186/s13072-015-0033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croll D, McDonald BA (2012) The accessory genome as a cradle for adaptive evolution in pathogens. PLoS Pathog 8: e1002608 10.1371/journal.ppat.1002608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandaubert J, Lowe RG, Soyer JL, Schoch CL, Van de Wouw AP, et al. (2014) Transposable element-assisted evolution and adaptation to host plant within the Leptosphaeria maculans-Leptosphaeria biglobosa species complex of fungal pathogens. BMC Genomics 15: 891 10.1186/1471-2164-15-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuma I, Isobe C, Hotta Y, Ibaragi K, Futamata N, et al. (2011) Multiple translocation of the AVR-Pita effector gene among chromosomes of the rice blast fungus Magnaporthe oryzae and related species. PLoS Pathog 7: e1002147 10.1371/journal.ppat.1002147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma LJ, van der Does HC, Borkovich KA, Coleman JJ, Daboussi MJ, et al. (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464: 367–373. 10.1038/nature08850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akagi Y, Akamatsu H, Otani H, Kodama M (2009) Horizontal chromosome transfer, a mechanism for the evolution and differentiation of a plant-pathogenic fungus. Eukaryot Cell 8: 1732–1738. 10.1128/EC.00135-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friesen TL, Stukenbrock EH, Liu Z, Meinhardt S, Ling H, et al. (2006) Emergence of a new disease as a result of interspecific virulence gene transfer. Nat Genet 38: 953–956. 10.1038/ng1839 [DOI] [PubMed] [Google Scholar]
- 10.Coleman JJ, Rounsley SD, Rodriguez-Carres M, Kuo A, Wasmann CC, et al. (2009) The genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS genetics 5: e1000618 10.1371/journal.pgen.1000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noguchi MT, Yasuda N, Fujita Y (2006) Evidence of Genetic Exchange by Parasexual Recombination and Genetic Analysis of Pathogenicity and Mating Type of Parasexual Recombinants in Rice Blast Fungus, Magnaporthe oryzae. Phytopathology 96: 746–750. 10.1094/PHYTO-96-0746 [DOI] [PubMed] [Google Scholar]
- 12.O'Connell RJ, Thon MR, Hacquard S, Amyotte SG, Kleemann J, et al. (2012) Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature genetics 44: 1060–1065. 10.1038/ng.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schilling L, Matei A, Redkar A, Walbot V, Doehlemann G (2014) Virulence of the maize smut Ustilago maydis is shaped by organ-specific effectors. Molecular plant pathology 15: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stergiopoulos I, de Wit PJ (2009) Fungal effector proteins. Annu Rev Phytopathol 47: 233–263. 10.1146/annurev.phyto.112408.132637 [DOI] [PubMed] [Google Scholar]
- 15.Torreblanca J, Stumpferl S, Basse CW (2003) Histone deacetylase Hda1 acts as repressor of the Ustilago maydis biotrophic marker gene mig1. Fungal Genet Biol 38: 22–32. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Kief J, Auffarth K, Farfsing JW, Mahlert M, et al. (2008) The Ustilago maydis Cys2His2-type zinc finger transcription factor Mzr1 regulates fungal gene expression during the biotrophic growth stage. Mol Microbiol 68: 1450–1470. 10.1111/j.1365-2958.2008.06244.x [DOI] [PubMed] [Google Scholar]
- 17.Heimel K, Scherer M, Vranes M, Wahl R, Pothiratana C, et al. (2010) The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis. PLoS Pathog 6: e1001035 10.1371/journal.ppat.1001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zahiri A, Heimel K, Wahl R, Rath M, Kamper J (2010) The Ustilago maydis forkhead transcription factor Fox1 is involved in the regulation of genes required for the attenuation of plant defenses during pathogenic development. Mol Plant Microbe Interact 23: 1118–1129. 10.1094/MPMI-23-9-1118 [DOI] [PubMed] [Google Scholar]
- 19.Wahl R, Zahiri A, Kamper J (2010) The Ustilago maydis b mating type locus controls hyphal proliferation and expression of secreted virulence factors in planta. Mol Microbiol 75: 208–220. 10.1111/j.1365-2958.2009.06984.x [DOI] [PubMed] [Google Scholar]
- 20.IpCho SV, Tan KC, Koh G, Gummer J, Oliver RP, et al. (2010) The transcription factor StuA regulates central carbon metabolism, mycotoxin production, and effector gene expression in the wheat pathogen Stagonospora nodorum. Eukaryot Cell 9: 1100–1108. 10.1128/EC.00064-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soyer JL, Hamiot A, Ollivier B, Balesdent MH, Rouxel T, et al. (2015) The APSES transcription factor LmStuA is required for sporulation, pathogenic development and effector gene expression in Leptosphaeria maculans. Mol Plant Pathol 16: 1000–1005. 10.1111/mpp.12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang G, Wang H, Chou S, Nie X, Chen J, et al. (2006) Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci U S A 103: 12813–12818. 10.1073/pnas.0605270103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michielse CB, Studt L, Janevska S, Sieber CM, Arndt B, et al. (2015) The global regulator FfSge1 is required for expression of secondary metabolite gene clusters but not for pathogenicity in Fusarium fujikuroi. Environmental microbiology 17: 2690–2708. 10.1111/1462-2920.12592 [DOI] [PubMed] [Google Scholar]
- 24.Jonkers W, Dong Y, Broz K, Kistler HC (2012) The Wor1-like protein Fgp1 regulates pathogenicity, toxin synthesis and reproduction in the phytopathogenic fungus Fusarium graminearum. PLoS pathogens 8: e1002724 10.1371/journal.ppat.1002724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown DW, Busman M, Proctor RH (2014) Fusarium verticillioides SGE1 is required for full virulence and regulates expression of protein effector and secondary metabolite biosynthetic genes. Mol Plant Microbe Interact 27: 809–823. 10.1094/MPMI-09-13-0281-R [DOI] [PubMed] [Google Scholar]
- 26.Michielse CB, van Wijk R, Reijnen L, Manders EM, Boas S, et al. (2009) The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog 5: e1000637 10.1371/journal.ppat.1000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michielse CB, Becker M, Heller J, Moraga J, Collado IG, et al. (2011) The Botrytis cinerea Reg1 protein, a putative transcriptional regulator, is required for pathogenicity, conidiogenesis, and the production of secondary metabolites. Mol Plant Microbe Interact 24: 1074–1085. 10.1094/MPMI-01-11-0007 [DOI] [PubMed] [Google Scholar]
- 28.Okmen B, Collemare J, Griffiths S, van der Burgt A, Cox R, et al. (2014) Functional analysis of the conserved transcriptional regulator CfWor1 in Cladosporium fulvum reveals diverse roles in the virulence of plant pathogenic fungi. Molecular microbiology 92: 10–27. 10.1111/mmi.12535 [DOI] [PubMed] [Google Scholar]
- 29.Santhanam P, Thomma BP (2013) Verticillium dahliae Sge1 differentially regulates expression of candidate effector genes. Mol Plant Microbe Interact 26: 249–256. 10.1094/MPMI-08-12-0198-R [DOI] [PubMed] [Google Scholar]
- 30.Mirzadi Gohari A, Mehrabi R, Robert O, Ince IA, Boeren S, et al. (2014) Molecular characterization and functional analyses of ZtWor1, a transcriptional regulator of the fungal wheat pathogen Zymoseptoria tritici. Mol Plant Pathol 15: 394–405. 10.1111/mpp.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Zhai S, Zhang H, Zuo R, Wang J, et al. (2014) Shared and distinct functions of two Gti1/Pac2 family proteins in growth, morphogenesis and pathogenicity of Magnaporthe oryzae. Environmental microbiology 16: 788–801. 10.1111/1462-2920.12204 [DOI] [PubMed] [Google Scholar]
- 32.Tollot M, Assmann D, Becker C, Altmuller J, Dutheil JY, et al. (2016) The WOPR Protein Ros1 Is a Master Regulator of Sporogenesis and Late Effector Gene Expression in the Maize Pathogen Ustilago maydis. PLoS Pathog 12: e1005697 10.1371/journal.ppat.1005697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cain CW, Lohse MB, Homann OR, Sil A, Johnson AD (2012) A conserved transcriptional regulator governs fungal morphology in widely diverged species. Genetics 190: 511–521. 10.1534/genetics.111.134080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt SM, Houterman PM, Schreiver I, Ma L, Amyotte S, et al. (2013) MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum. BMC genomics 14: 119 10.1186/1471-2164-14-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vega-Bartol JJ, Martin-Dominguez R, Ramos B, Garcia-Sanchez MA, Diaz-Minguez JM (2011) New virulence groups in Fusarium oxysporum f. sp. phaseoli: the expression of the gene coding for the transcription factor ftf1 correlates with virulence. Phytopathology 101: 470–479. 10.1094/PHYTO-09-10-0252 [DOI] [PubMed] [Google Scholar]
- 36.Nino-Sanchez J, Tello V, Casado-Del Castillo V, Thon MR, Benito EP, et al. (2015) Gene expression patterns and dynamics of the colonization of common bean (Phaseolus vulgaris L.) by highly virulent and weakly virulent strains of Fusarium oxysporum. Front Microbiol 6: 234 10.3389/fmicb.2015.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonkers W, Xayamongkhon H, Haas M, Olivain C, van der Does HC, et al. (2014) EBR1 genomic expansion and its role in virulence of Fusarium species. Environmental microbiology 16: 1982–2003. 10.1111/1462-2920.12331 [DOI] [PubMed] [Google Scholar]
- 38.Berger MF, Philippakis AA, Qureshi AM, He FS, Estep PW 3rd, et al. (2006) Compact, universal DNA microarrays to comprehensively determine transcription-factor binding site specificities. Nat Biotechnol 24: 1429–1435. 10.1038/nbt1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Georges AB, Benayoun BA, Caburet S, Veitia RA (2010) Generic binding sites, generic DNA-binding domains: where does specific promoter recognition come from? FASEB J 24: 346–356. 10.1096/fj.09-142117 [DOI] [PubMed] [Google Scholar]
- 40.Adams CC, Workman JL (1995) Binding of disparate transcriptional activators to nucleosomal DNA is inherently cooperative. Mol Cell Biol 15: 1405–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Zhang T, Yan M, Ding J, Chen J (2014) Crystal structure of the WOPR-DNA complex and implications for Wor1 function in white-opaque switching of Candida albicans. Cell Res 24: 1108–1120. 10.1038/cr.2014.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohse MB, Zordan RE, Cain CW, Johnson AD (2010) Distinct class of DNA-binding domains is exemplified by a master regulator of phenotypic switching in Candida albicans. Proc Natl Acad Sci U S A 107: 14105–14110. 10.1073/pnas.1005911107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohse MB, Rosenberg OS, Cox JS, Stroud RM, Finer-Moore JS, et al. (2014) Structure of a new DNA-binding domain which regulates pathogenesis in a wide variety of fungi. Proc Natl Acad Sci U S A 111: 10404–10410. 10.1073/pnas.1410110111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alkahyyat F, Ni M, Kim SC, Yu JH (2015) The WOPR Domain Protein OsaA Orchestrates Development in Aspergillus nidulans. PLoS One 10: e0137554 10.1371/journal.pone.0137554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt SM, Brouwer S, Lukasiewicz J, Fokkens L, van Dam P, et al. (submitted) The in planta transcriptomes of strains of Fusarium oxysporum specific for tomato or melon include genes for transcription factors required for full virulence.
- 46.van der Does HC, Duyvesteijn RG, Goltstein PM, van Schie CC, Manders EM, et al. (2008) Expression of effector gene SIX1 of Fusarium oxysporum requires living plant cells. Fungal genetics and biology: FG & B 45: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 47.Houterman PM, Ma L, van Ooijen G, de Vroomen MJ, Cornelissen BJ, et al. (2009) The effector protein Avr2 of the xylem-colonizing fungus Fusarium oxysporum activates the tomato resistance protein I-2 intracellularly. The Plant journal: for cell and molecular biology 58: 970–978. [DOI] [PubMed] [Google Scholar]
- 48.Gawehns F, Houterman PM, Ichou FA, Michielse CB, Hijdra M, et al. (2014) The Fusarium oxysporum effector Six6 contributes to virulence and suppresses I-2-mediated cell death. Molecular plant-microbe interactions: MPMI 27: 336–348. 10.1094/MPMI-11-13-0330-R [DOI] [PubMed] [Google Scholar]
- 49.Gawehns F, Ma L, Bruning O, Houterman PM, Boeren S, et al. (2015) The effector repertoire of Fusarium oxysporum determines the tomato xylem proteome composition following infection. Front Plant Sci 6: 967 10.3389/fpls.2015.00967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dam P, Fokkens L, Schmidt SM, Linmans JH, Kistler HC, et al. (2016) Effector profiles distinguish formae speciales of Fusarium oxysporum. Environ Microbiol. [DOI] [PubMed] [Google Scholar]
- 51.Schoffelmeer EA, Vossen JH, van Doorn AA, Cornelissen BJ, Haring MA (2001) FEM1, a Fusarium oxysporum glycoprotein that is covalently linked to the cell wall matrix and is conserved in filamentous fungi. Mol Genet Genomics 265: 143–152. [DOI] [PubMed] [Google Scholar]
- 52.Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlaardingerbroek I, Beerens B, Schmidt SM, Cornelissen BJ, Rep M (2016) Dispensable chromosomes in Fusarium oxysporum f.sp lycopersici. Mol Plant Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Priebe S, Kreisel C, Horn F, Guthke R, Linde J (2015) FungiFun2: a comprehensive online resource for systematic analysis of gene lists from fungal species. Bioinformatics 31: 445–446. 10.1093/bioinformatics/btu627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michielse CB, van Wijk R, Reijnen L, Cornelissen BJ, Rep M (2009) Insight into the molecular requirements for pathogenicity of Fusarium oxysporum f. sp. lycopersici through large-scale insertional mutagenesis. Genome biology 10: R4 10.1186/gb-2009-10-1-r4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu JH, Keller N (2005) Regulation of secondary metabolism in filamentous fungi. Annu Rev Phytopathol 43: 437–458. 10.1146/annurev.phyto.43.040204.140214 [DOI] [PubMed] [Google Scholar]
- 57.Studt L, Schmidt FJ, Jahn L, Sieber CM, Connolly LR, et al. (2013) Two histone deacetylases, FfHda1 and FfHda2, are important for Fusarium fujikuroi secondary metabolism and virulence. Applied and environmental microbiology 79: 7719–7734. 10.1128/AEM.01557-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connolly LR, Smith KM, Freitag M (2013) The Fusarium graminearum histone H3 K27 methyltransferase KMT6 regulates development and expression of secondary metabolite gene clusters. PLoS genetics 9: e1003916 10.1371/journal.pgen.1003916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webster RH, Sil A (2008) Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proc Natl Acad Sci U S A 105: 14573–14578. 10.1073/pnas.0806221105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nino-Sanchez J, Casado-Del Castillo V, Tello V, de Vega-Bartol JJ, Ramos B, et al. (2016) The FTF gene family regulates virulence and expression of SIX effectors in Fusarium oxysporum. Mol Plant Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghag SB, Shekhawat UK, Ganapathi TR (2014) Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol J 12: 541–553. 10.1111/pbi.12158 [DOI] [PubMed] [Google Scholar]
- 62.Son H, Seo YS, Min K, Park AR, Lee J, et al. (2011) A phenome-based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum. PLoS Pathog 7: e1002310 10.1371/journal.ppat.1002310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo L, Zhao G, Xu JR, Kistler HC, Gao L, et al. (2016) Compartmentalized gene regulatory network of the pathogenic fungus Fusarium graminearum. New Phytol 211: 527–541. 10.1111/nph.13912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Does HC, Lievens B, Claes L, Houterman PM, Cornelissen BJ, et al. (2008) The presence of a virulence locus discriminates Fusarium oxysporum isolates causing tomato wilt from other isolates. Environmental microbiology 10: 1475–1485. 10.1111/j.1462-2920.2007.01561.x [DOI] [PubMed] [Google Scholar]
- 65.Takken FL, Van Wijk R, Michielse CB, Houterman PM, Ram AF, et al. (2004) A one-step method to convert vectors into binary vectors suited for Agrobacterium-mediated transformation. Curr Genet 45: 242–248. 10.1007/s00294-003-0481-5 [DOI] [PubMed] [Google Scholar]
- 66.Rep M, van der Does HC, Meijer M, van Wijk R, Houterman PM, et al. (2004) A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Molecular microbiology 53: 1373–1383. 10.1111/j.1365-2958.2004.04177.x [DOI] [PubMed] [Google Scholar]
- 67.Berger MF, Badis G, Gehrke AR, Talukder S, Philippakis AA, et al. (2008) Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell 133: 1266–1276. 10.1016/j.cell.2008.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Philippakis AA, Qureshi AM, Berger MF, Bulyk ML (2008) Design of compact, universal DNA microarrays for protein binding microarray experiments. J Comput Biol 15: 655–665. 10.1089/cmb.2007.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam KN, van Bakel H, Cote A, vdV A., Hughes TR (2011) Sequence specificity is obtained from the majority of modular C2H2 zinc-finger arrays. Nucleic Acids Res 39: 4680–4690. 10.1093/nar/gkq1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weirauch MT, Cote A, Norel R, Annala M, Zhao Y, et al. (2013) Evaluation of methods for modeling transcription factor sequence specificity. Nat Biotechnol 31: 126–134. 10.1038/nbt.2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Narasimhan K, Lambert SA, Yang AW, Riddell J, Mnaimneh S, et al. (2015) Mapping and analysis of Caenorhabditis elegans transcription factor sequence specificities. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weirauch MT, Yang A, Albu M, Cote AG, Montenegro-Montero A, et al. (2014) Determination and inference of eukaryotic transcription factor sequence specificity. Cell 158: 1431–1443. 10.1016/j.cell.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14: 1188–1190. 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 75.Sievers F, Higgins DG (2014) Clustal Omega, accurate alignment of very large numbers of sequences. Methods Mol Biol 1079: 105–116. 10.1007/978-1-62703-646-7_6 [DOI] [PubMed] [Google Scholar]
- 76.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973. 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 78.Chen DC, Yang BC, Kuo TT (1992) One-step transformation of yeast in stationary phase. Curr Genet 21: 83–84. [DOI] [PubMed] [Google Scholar]
- 79.Houterman PM, Cornelissen BJ, Rep M (2008) Suppression of plant resistance gene-based immunity by a fungal effector. PLoS pathogens 4: e1000061 10.1371/journal.ppat.1000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic trees of the protein sequences of the different transcription factor families, including homologs in Fol, other F. oxysporum ff. spp. and other Fusarium species. A) Family of aTf4 (FOXG_14201), a C2H2 zinc finger transcription factor—this transcription factor has no additional accessory homologs in Fol. B) aTf3 (FOXG_17266), a C2H2 zinc finger transcription factor with no homologs on the core genome, and only two homologs in accessory regions in other formae speciales. C) Family of aTf6 (FOXG_14211), a C2H2 zinc finger transcription factor—this transcription factor has no additional accessory homologs in Fol. D) Family of aTf7 (FOXG_14275), a C2H2 zinc finger transcription factor. This transcription factor has seven additional accessory homologs in Fol4287. E) Protein family of aTf1 (Ftf1; FOXG_14257, FOXG_17458, FOXG_16414) of Zn(2)Cys(6) zinc finger transcription factors. This transcription factor has seven additional accessory homologs in Fol4287. F) Family of aTf8 (FOXG_14277), a Zn(2)Cys(6) zinc finger transcription factor. This transcription factor has five additional accessory homologs in Fol4287. G) Family of aTf9 (FOXG_14274, FOXG_14137, FOXG_14220) of bZIP leucine zipper transcription factors. This family has seven additional accessory homologs in Fol4287. H) Family of aTf2 (FOXG_17260), a forkhead transcription factor with one homolog on the core genome and one homolog on the accessory chromosomes. Only three other forkhead transcription factor genes are present in the Fol4287 genome. I) Family of aTf5 (FOXG_14230), a bZIP leucine zipper transcription factor—this transcription factor has no additional accessory homologs in Fol.
(PDF)
In-frame potential startcodons (ATG) are indicated with red arrows and are bold and underlined, other ATG codons are bold. Red bases indicate single basepair indels. The following genes have a ‘long’ predicted ORF, according to the Broad annotation (the number refers to the FOXG number of the Broad annotation): 09390, cTF1, core genome; 17458, pathogenicity chromosome, used for overexpression & RNAseq; 14257, pathogenicity chromosome, 14422, accessory chromosome 15; 15059, accessory region on chromosome 1. The remaining genes have a shorter predicted ORF according to the Broad annotation, namely: 16414, pathogenicity chromosome; 17123, accessory chromosome 6, 17084, accessory chromosome 3/6, used for overexpression; 14000, accessory chromosome 3/6; 12589, accessory chromosome 3/6, 12539, accessory chromosome 3/6. The genes with a long predicted ORF start at the ATG indicated with red arrow number 1. All remaining genes have a 17 base pair deletion (compared to the core homolog cTF1/>09390), removing this ATG. Their predicted startcodon is the next in-frame ATG (red arrow 2). In addition, all homologs with the 17 bp deletion also have a more upstream stop codon– 300 bp upstream of the stopcodon in cTF1. This means that all ORFs starting at the position indicated by red arrow 2, start 117 bp later and stop 300 bp earlier than the long TF1 homologs, rendering ORFs of around 2800 bp. These genes we refer to as having a short ORF. The ORFs starting at the position indicated by red arrow 1 are around 3200 bp. These genes we refer to as having a long ORF.
(PDF)
For each family we predicted domain architecture for its members. We here show for each family, from the multiple sequence alignment used to generate its phylogeny (S1 Fig), those regions that correspond to a DNA binding domain. The background color of a residue corresponds to its conservation in the alignment, where darker colors indicate more conservation. Residues that are conserved in the seed alignment from Pfam and are present in at least two sequence are indicated in bold on top of the alignment. Residues that are conserved in the Pfam seed alignment but are not present in at least two sequences are in normal font. Gene names correspond to names and species or ff. spp. mentioned in Fig 2and S1 Fig. Core-encoded transcription factors of F. oxysporum (Fol4287), F. verticillioides, F. graminearum and F. solani are indicated in red. Accessory transcription factors from Fol tested for DNA binding specificity are indicated in bold.
(PDF)
A) The number of genes with one or more transcription factor DNA binding site (TF DBS) in the 1000 bp upstream of the predicted transcriptional start site, for the complete genome, and for a subset of genes (up- or down-regulated during infection). Boxes indicate a significant enrichment (p value <0.01 after Bonferroni correction). The motif found in effector gene promoters earlier (aacTGCCGa and overlapping with the Tf1 DNA binding site has been included. For all categories the total number of genes is given (ALL, green), the number of core genes (CORE, blue) and the number of accessory genes (purple). Accessory genes are subdivided in total (ACC), accessory genes not on the pathogenicity chromosome (no14) and the accessory genes on the pathogenicity chromosome (only_14). B) As A, but for the 1000 bp downstream the ATG. C) As A, but only for the Tf1 DNA binding site (≥3 occurrences), the motif found in the promoters of effector genes, overlapping with the Tf1 DNA binding site (≥1 occurrences) and the Sge1 DNA binding site (≥1 occurrences). The following categories are additional: genes UP or DOWN regulated in the aTF1, cTF1 or SGE1 overexpressors, genes for small secreted proteins, genes with a MIMP in their promoter, genes coding for proteins found in xylem sap of infected plants. D) As C, but for the 1000 bp downstream the ATG.
(PDF)
A) Estimated percentage, by visual inspection, of GFP-fluorescent spores in independent transformants of different transcription factor overexpression constructs. x: this transformant does not exist (misnumbered), *: of the background strain (Psix1GFP—WT) the same transformant was tested multiple times, each number represents therefore a replicate instead of a different transformant. B) Relative expression of different transcription factor genes in WT and their respective overexpressors as determined by Q-RT-PCR. Relative expression is calculated as TF expression/expression of EF1α. Minimally three independent transformants were tested per transcription factor. Error bars indicate the standard deviation of two technical replicates. C) Growth rate of the different transcription factor overexpressors. Minimally three independent transformants were tested per transcription factor, on both rich medium (potato dextrose agar, PDA, top panel) and complete minimal medium (Czapek Dox Agar, CDA, bottom panel). Stars indicate significant differences compared to the background strain (T-test, p<0.01). Error bars indicate the standard deviation of minimally two independent replicates. D) Bioassay on susceptible tomato plants. Minimally three independent transformants were tested per transcription factor. Control treatments were: mock, WT (this is the WT used to make the background strain, Fol007), Psix1GFP (the background strain, WT + reporter construct Psix1GFP), Psix1GFP + RFP (the background strain plus a constitutively expressed RFP construct). Top panel: average plant weight (gram) 21 dpi. Error bars indicate standard deviation. Stars indicate a significant difference from the background strain (Psix1GFP, T-test, p<0.01). Bottom panel: disease index (0–4 arbitrary units) 21 dpi.
(PDF)
A) Top three Venn diagrams show the overlap of sets of differentially expressed genes between two independent transformants of each transcription factor. The overlapping genes are depicted in red and these are the genes considered for all further analyses. Lower six Venn diagrams: overlap between target genes in the different overexpressors, up-regulated (left three diagrams) or down-regulated (right three diagrams). B&C) Number of up- or down-regulated genes in different subcategories. Significant enrichment or depletion in each subcategory was tested with a hypergeometric test, P value < 0.01 after Bonferroni correction. Orange cells: enrichment, blue cells: depletion. Columns: whole genome (all genes), planta UP (genes up-regulated during infection), aTF1 UP, cTF1 UP, SGE1 UP (genes up-regulated in the aTF1, cTF1 or SGE1 overexpressors, a1c1S1 UP (genes up-regulated in all three overexpressors), planta DOWN (genes down-regulated during infection), aTF1 DOWN, cTF1 DOWN, SGE1 DOWN (genes down-regulated in the aTF1, cTF1 or SGE1 overexpressors, a1c1S1 DOWN (genes down-regulated in all three overexpressors), MIMP (genes with a miniature impala transposable element (MIMP) in their promoter), XS proteins (genes of which the protein product was found in the xylem sap of infected tomato plants [34]). Rows: all (all genes), core (all genes located on the core genome), pathogenicity chr. (all genes on the pathogenicity chromosome), other accessory chr. (all accessory genes, except those on the pathogenicity chromosome), in planta UP all (all genes up-regulated during infection), UP core (genes located on core chromosomes and up-regulated during infection), UP pathogenicity chr. (genes located on the pathogenicity chromosome and up-regulated during infection), UP accessory chr. (genes on the accessory regions, except those on the pathogenicity chromosome, and up-regulated during infection), in planta DOWN (all genes down-regulated during infection), SIX genes (all SIX genes), XS proteins (genes of which the protein product was found in the xylem sap of infected tomato plants [34]), XS proteins w/o SIX proteins (genes coding for xylem sap proteins minus the SIX genes), mimp (genes with a MIMP in their promoter), mimp on accessory chr. (genes on the accessory regions with a MIMP in their promoter), mimp on pathogenicity chr. (genes on the pathogenicity chromosome with a MIMP in their promoter), mimp on core (genes on the core chromosomes with a MIMP in their promoter), mimp w/o SIX genes (genes with a MIMP in their promoter, without the SIX genes), mimp on path. chr. w/o SIX (genes on the pathogenicity chromosome with a MIMP in their promoter, without the SIX genes). D) Number of up- or down-regulated transposable element transcripts in different subcategories. All sequencing reads from the overexpressors and reads from infected plant material were mapped to a fasta file in which the sequence of each repetitive element, plus each previously annotated transposable element, is present once. The number of reads was normalized to the number of reads mapped uniquely to the whole genome for each sample. The number of reads mapping to each different sequence was counted and differentially expressed sequences were called. Significant enrichments or depletions were tested with a hypergeometric test, P value < 0.01 after Bonferroni correction. Orange cells: enrichment, blue cells: depletion. Columns: ALL (all genes or transposable elements), UP (up-regulated genes or transposable elements), DOWN (down-regulated genes or transposable elements), aTF1A & aTF1E (two independent aTF1 overexpressors), cTF1C & cTF1D (two independent cTF1 overexpressors), SGE15 &SGE1M (two independent SGE1 overexpressors), in planta (Fol sampled from infected plant material). Accessory; all genes located on the accessory regions, TE: transposable elements. Note that the reads mapping to a single sequence may be derived from many different locations on the genome, so each sequence represents ‘net’ expression. Because most transposable elements are located on accessory chromosomes, comparisons were made to those regions specifically. The portion of repetitive/transposon sequences down-regulated in the different transformants is very similar to the portion of accessory genes down-regulated. The number of transposons up-regulated in the different transformants is lower than the portion of genes up-regulated on the accessory chromosomes. Both may be due to inclusion of transposable elements in the large scale gene repression on the accessory chromosomes, given that we look at the net expression for each sequence. Also during infection—tested with a strain that does not show the large accessory gene repression—no increased up-regulation of transposon transcripts is observed.
(PDF)
Relative expression was determined by Q-RT-PCR, and calculated as TF expression /expression of EF1α. Two independent transformants were tested per transcription factor. Plants were infected with strain Fol007 and roots were harvested for RNA extraction 9 dpi. Error bars indicate the standard error of two (during infection) or three (all other samples) independent biological replicates.
(PDF)
A) Relative expression of aTF1, GFP and SIX3 compared to expression of the reference gene EF1α determined by Q-RT-PCR. Expression was measured in nine different independent aTF1 overexpressors (a, b, c, e, f, g, j, k and o) and in the background strain (Psix1GFP). aTF1 is expressed from the endogenous locus and–in the overexpressors–from an additional overexpression construct. GFP is expressed by the SIX1 promoter, from the original locus. SIX1 and SIX3 are representative effector genes. Relative expression is calculated as gene expression/expression of EF1α. Error bars indicate standard deviation. B) clustering of differentially expressed genes (Padjusted < 0.01) after expression of aTF1, cTF1 or SGE1 from the FEM1 promoter, by log2 fold change values for each differentially expressed gene. The log2 fold change values of genes that are not significantly different from the control are set to zero. Two independent transformants were studied per transcription factor, resulting in six independent conditions. The tree reflects the similarity between the transcriptional changes in each sample.
(PDF)
Heatmap of differentially expressed genes during infection or after in aTF1, cTF1 or SGE1 overexpressors, divided per subgenome (the pathogenicity chromosome, other accessory regions and core genome). Displayed is the log2 fold change for each differentially expressed gene (rows), with yellow indicating up-regulation compared to the control (hues cover the log2 fold range from 0 to 5). Blue indicates down-regulation compared to the control (hues cover the log2 fold range from 0 to -5). The log2 fold change values of genes that are not significantly different from the control are set to zero. Each condition is a separate column, with two independent transformants (thus 2 columns) per transcription factor. The order of the rows reflects clustering of similar expression patterns. The first seven columns are the same as in Fig 8A, the eighth column is additional (column header highlighted in red). Wild type strain Fol4287 was used for in planta samples, and for Fol4287 samples from flask (compared to each other in the first column). Fol007 is a wild type strain very similar to Fol4287 and was used to create the background strain (Fol007 + Psix1GFP reporter construct) for the overexpressors. Overexpressors and their background strain were compared in column two to seven. The eighth column compares the two wild type strains to each other (Fol4287 and Fol007+Psix1GFP). The red bar highlights the group of genes that is expressed lower in Fol 4287 WT and in the overexpressors in Fol 007 + Psix1GFP background, compared to Fol007 + Psix1GFP.
(PDF)
Expression (of the background strain, top row, green) or the difference in expression with the background strain (of the overexpressors, six bottom rows, different shades of red) in RPKM or ΔRPKM (log10 scale). On the x-axis, the genes of SC 22, SC 36 and SC 51 are plotted, in the same order as they appear on the supercontig (SC). Only positive numbers are shown for the overexpressors (ΔRPKM)–down-regulated genes are not visible. No distinction has been made between significant and non-significant up-regulation—all differences are shown. The second and third rows show two independent aTF1 overexpressors (dark red), the fourth and fifth rows show two independent cTF1 overexpressors (bright red), the sixth and seventh rows show two independent SGE1 overexpressors (orange). The dashed box indicates the region that is up-regulated in one cTF1 overexpressor and one SGE1 overexpressor.
(PDF)
All sequencing reads from the overexpressors and reads from infected plant material were mapped to a fasta file where the sequence of each repetitive element, plus all each previously annotated transposable element, is present once. The number of reads was normalized to the number of reads mapped uniquely to the whole genome for each sample. In the graph, the total number of reads assigned to transposable elements per 20 million reads uniquely mapped to the reference genome are shown. Of each transcription factor overexpressor (aTF1, cTF1 or SGE1) two independent transformants are shown. No consistent significant effects on overall transposon expression were observed in any of the overexpressors. However, the total amount of transposon-derived reads during infection was reduced.
(PDF)
Bioassay on susceptible tomato plants. Two independent transformants were tested for both ectopic and in locus integration of the SGE1 deletion construct in the aTF1 overexpressor (aTF1 OE ectopic and aTF1 Δsge1, respectively). Control treatments were: mock, Fol4287 (WT), Δsge1 (SGE1 deletion mutant in Fol4287), aTF1 OE (aTF1 overexpressor in Fol4287). Left panel: average plant weight (gram) 21 dpi. Error bars indicate standard deviation. Right panel: disease index (0–4 arbitrary units) 21 dpi.
(PDF)
This file contains an overview of the transcription factors mentioned in this study, an overview of the results of the DNA binding assay, an overview of DNA binding site occurrences, overviews of genes up- or down-regulated in aTF1, cTF1 and SGE1 overexpressors, an overview of genes with a MIMP within 2 kb upstream of the ATG, genes of which the protein product is detected in xylem sap and genes up- or down-regulated in planta, a list of the primers used in this study and the mapped read counts + RPKM values for all RNA-seq samples.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.