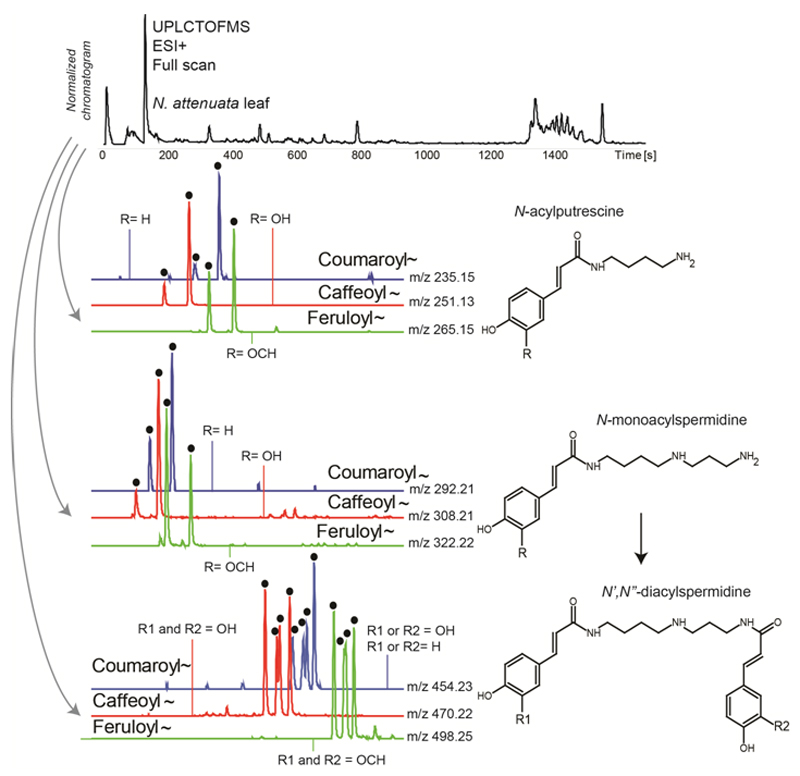
Figure 1. Mass spectrometry-based metabolomics of main phenolamides in N. attenuata leaves.
Phenolamides can readily be analyzed by ultra-high performance liquid chromatography coupled with a mass spectrometer. A representative UHPLC-time-of-flight-mass spectrometry (TOFMS) full scan chromatogram recorded in the positive ionization mode for an extract of an herbivory-induced leaf of Nicotina attenuata is shown. Coumaroyl- (m/z 147.05), caffeoyl- (m/z 163.04), or feruloyl (m/z 177.05) moieties resulting from the cleavage from different core molecules can, for example, be queried rapidly to compute extracted ion currents from the chromatogram. Specific m/z signals corresponding to either coumaroyl-, caffeoyl- and feruloyl-containing mono-acylated putrescine molecules or mono- and diacylated spermidines (N’,N”-coumaroyl,caffeoylspermidine, N’,N”-dicaffeoylspermidine, N’,N”-diferuloylspermidine) can be queried to reveal phenolamide peaks (highlighted with black dots). Representative structures are shown.