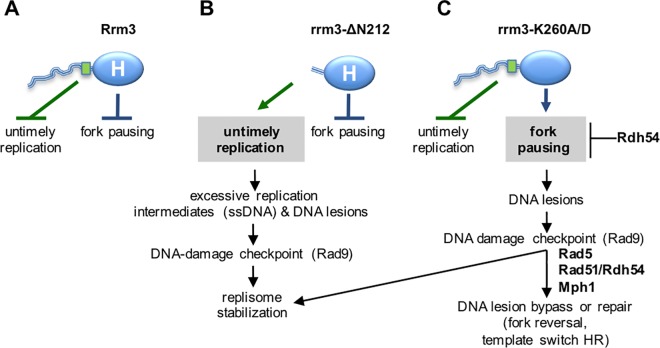
Fig 6. Rrm3 performs two genetically and physically separable functions during DNA replication.
(A) Rrm3 controls DNA synthesis via residues 186–212 (green rectangle) in its disordered N-terminal tail (blue line). Rrm3 associates with a subset of early and late-initiating origins of replication in G1 and S phase. Through binding Orc5 and maintaining association of Rrm3 with origins during replication stress, we propose that the N-terminal tail of Rrm3 may control DNA synthesis by controlling some origin activity to prevent untimely replication during replication stress. The N-terminal tail may also play a role in unperturbed cells as indicated by constitutive checkpoint activation in its absence. Independently of its N-terminal tail, Rrm3 associates with the replisome and utilizes its ATPase/helicase activity (blue oval labeled H) to facilitate fork progression through replication blocks. (B) Deleting the N-terminal tail of Rrm3 disrupts Orc5 binding and association with origins and causes enhanced DNA synthesis and S phase progression in HU, as well as accumulation of point mutations in normal S-phase and in HU. Excessive accumulation of replication intermediates and ensuing DNA lesions are the most probable cause of DNA-damage checkpoint activation in these cells. Despite loss of the N-terminal function, the ATPase/helicase-dependent function of Rrm3 in fork progression through blockages is intact, suggesting that Rrm3 can be recruited to replisomes independently of the N-terminal tail. (C) Disrupting the ATPase/helicase activity of Rrm3 maintains control over DNA synthesis, but forks are unable to progress through replication blocks, leading to DNA lesions that require Rad5, Rad51, Rdh54, and Mph1 for bypass or repair and activate the DNA damage checkpoint. In contrast to loss of the Orc5-binding site, a small increase in GCRs is observed in addition to point mutations, indicating the formation of different types of DNA lesions in the ATPase/helicase mutant, most likely DNA breaks.