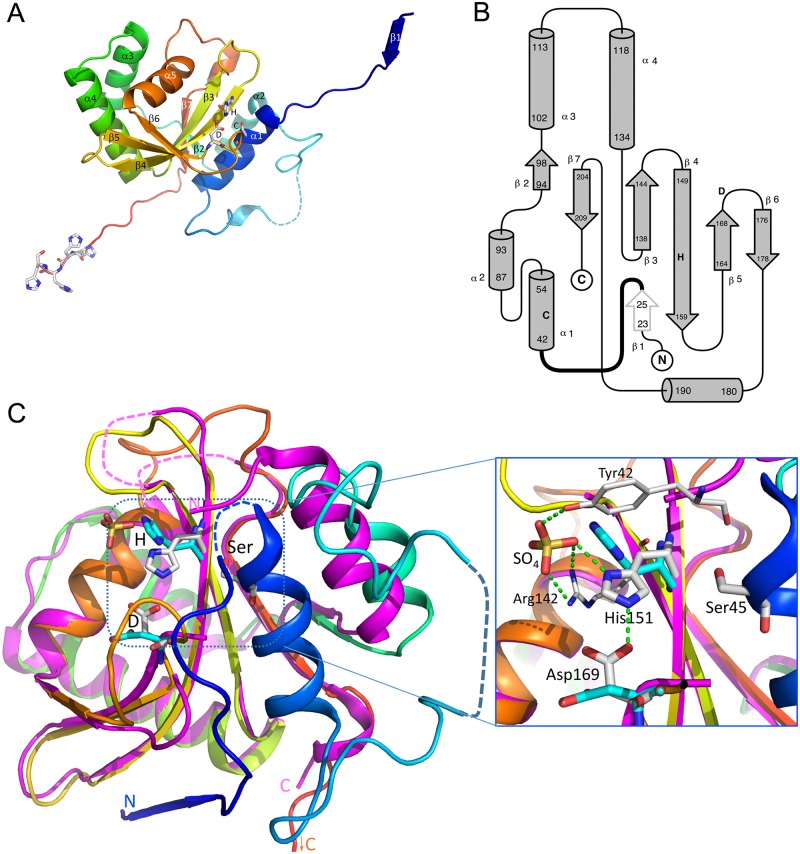
Fig 2. Structure of GtgE(17–214, Δ33–40, C45S).
(A) Cartoon representation with the active site residues shown in a stick representation and marked with one-letter code. Position of Ser45 is marked with letter C to indicate that this residue is a cysteine in the wild type GtgE. The molecule is colored in rainbow, from blue at the N-terminus to red at the C-terminus. The disordered segment is shown by a dashed line. The extended termini contact symmetry-related molecules in the crystal. The strand ß1 extends ß-sheet of the neighboring molecule. The catalytic residues and the four C-terminal histidines are shown in a stick mode. Secondary structure elements are labeled. This and subsequent figures were prepared with PyMol (www.pymol.org). (B) The topology diagram of GtgE. The location of catalytic residues is marked with letters C, H and D. (C) The superposition of GtgE (colored rainbow, this work) and the GtgE(79–214) fragment (magenta, PDB code 4MI7, [22]). The ~20 swapped N-terminal residues of the symmetry related molecule are depicted here to show the structure of an intact GtgE (see Fig 3C). The N- and C-termini are marked, the active site residues, Ser45 (Cys45 in wild type GtgE), His151 and Asp169 are shown in stick mode and colored white for GtgE and cyan for GtgE fragment. The inserts on the right shows the expanded view of the active site as observed in our structure. A sulfate molecule is bound near the catalytic histidine and forms hydrogen bonds with its ND1 atom as well as with Tyr42 and Arg142 sidechains. In the presence of a substrate the His151 sidechain would flip by 180° to form hydrogen bond between its ND1 nitrogen and SG of Cys45. Green dashed line indicates the hydrogen bond between His151 and Asp169 observed in the GtgE but not in the GtgE(79–214) fragment.