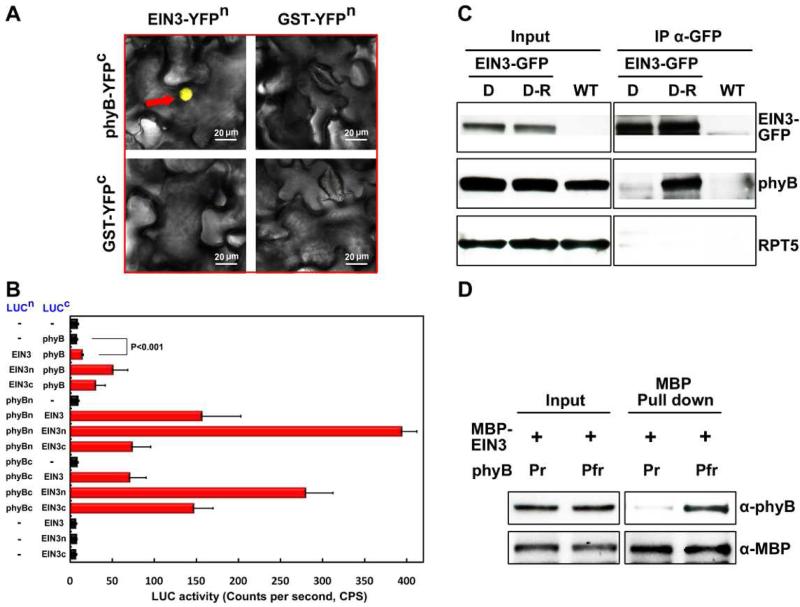
Figure 1. Photoreceptor phyB directly binds to the transcription factor EIN3 in a red light-dependent manner.
(A) BiFC assay detecting the interactions of phyB and EIN3. Full-length EIN3 and phyB were fused to the split N-terminal or C-terminal fragment of YFP (YFPn or YFPc), respectively. GST was used as a negative control. Reconstituted functional YFP is indicated with a red arrow. Bar = 20 μm.
(B) LCI assay showing the interactions of EIN3 and phyB in planta. The full-length sequence, the N-terminal or C-terminal fragments of EIN3 (EIN3, EIN3n, EIN3c) and phyB (phyB, phyBn, phyBc) were fused to the split N-terminal and C-terminal fragments of luciferase (LUCn and LUCc). “–“ indicates for the empty vectors as negative controls. C.P.S indicates for counts per second. Mean ± s.d., n=5. Student's t test was used to determine statistical significance.
(C) CoIP assay to detect the phyB-EIN3 association. 35S:EIN3-GFP/ein3eil1 (EIN3-GFP) and Col-0 (WT) seedlings were grown on 1/2 MS medium with 10 μM ACC for 4 days in the dark, and then either maintained in the dark (D) or exposed to red light (D-R) for 30 min. Proteins were then extracted and immunoprecipitated using anti-GFP antibody and immunoblotted using anti-GFP, anti-phyB and anti-RPT5 antibodies.
(D) In vitro pull-down assay for determining the interaction of the inactive Pr or active Pfr form phyB with MBP-EIN3. Recombinant phyB, added with either phycocyanobilin (Pfr) or methanol alone (Pr), was pre-treated with 0.5 hr of red light and then pulled down with MBP-EIN3 proteins. Anti-MBP and anti-phyB were used for immunoblot analysis.