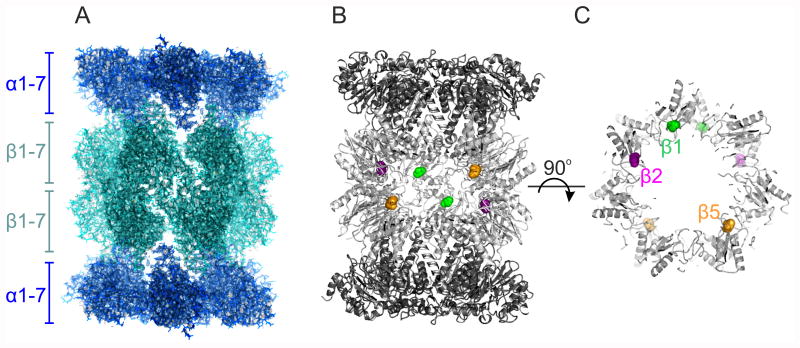
Figure 1.
The cryo-EM structure of the Plasmodium falciparum 20S proteasome (EMDB‐3231, PDB 5FMG). (A) Overall view of the 20S proteasome along its two-fold axis, with the location of its α and β hetero-heptameric rings indicated. The back surface of the barrel shaped structure was clipped for clarity. (B) Location of the proteolytic sites within the proteasome inner chamber, where the protein is represented as ribbons and the proteolytic active Thr1 of β1 (green), β2 (magenta) and β5 (orange) are represented as spheres. The front of the structure was clipped for clarity. (C) The same representation as for (B), but viewed along the proteasome long axis, and clipped to show the proteasome inner cavity. The Thr1 of the proteolytic active subunits are colour coded as in (B), but for clarity only those from the β subunit ring proximal to the viewport are labelled.