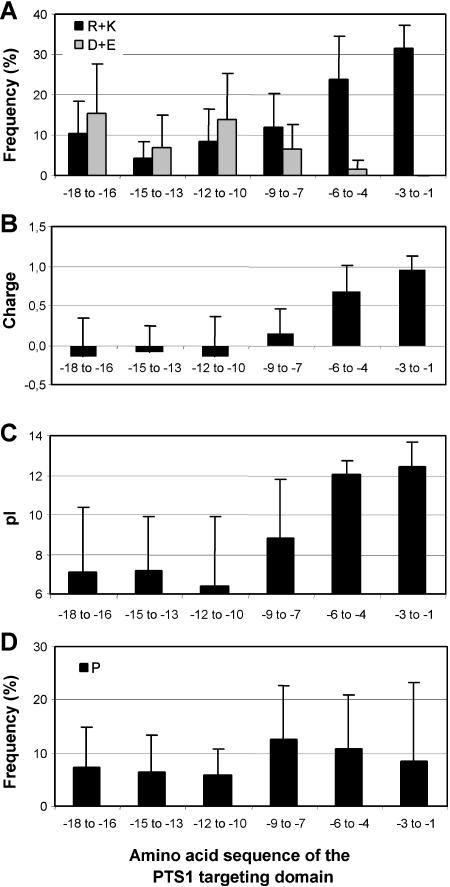
Figure 5.
Conserved properties of the PTS1 targeting domain. A, Relative content of basic (R + K) and acidic amino acids (D + E). B, Net charge, determined as the number of basic (R + K) minus acidic residues (D + E). C, pI. D, Relative content of P. The homologs of PTS1-targeted proteins were grouped according to their PTS1 tripeptide. Sequences of groups containing less than five sequences were analyzed together but sequences with unique C-terminal tripeptides were excluded. The amino acid composition of the C-terminal 18-mer was analyzed in groups of three amino acids. Apart from an enrichment in basic and P residues, the PTS1 domain is characterized by a low content of S-containing (C, M) and aromatic residues and a high content of hydrophobic residues, especially A and L, as well as hydroxylated amino acids (A + L, 19% in the C-terminal 18 amino acids; S + T, 15% between position −7 and −15; data not shown).