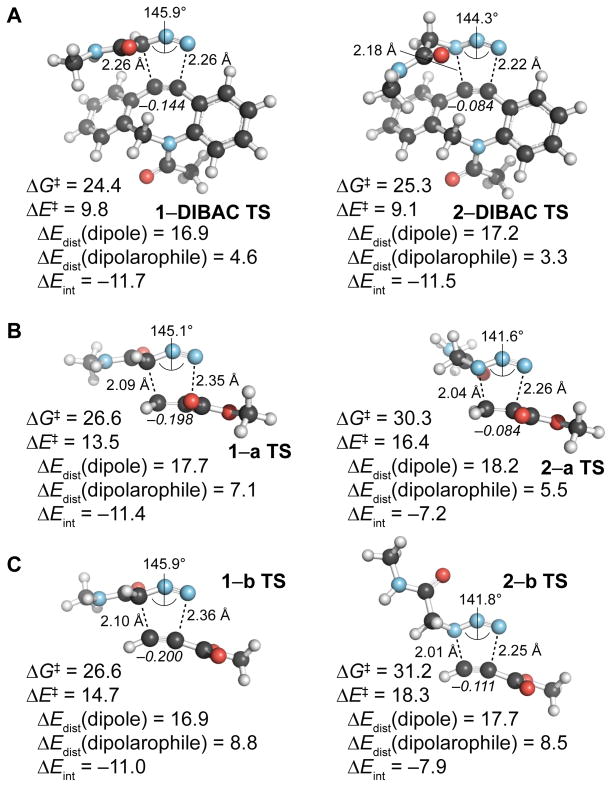
Figure 1.
Computational analysis of cycloadditions with N-methyl-2-diazoacetamide and N-methyl-2-azidoacetamide. Optimized geometries, activation energies, distortion-interactions energies, and changes in dipolarophile charge (italics) were calculated at the M06-2X/6-31+G(2d,p) level of theory. Energies (kcal/mol) and NBO charges on dipolarophiles (italics) include solvation corrections (water) on gas-phase geometries using IEFPCM model (radii=UFF).