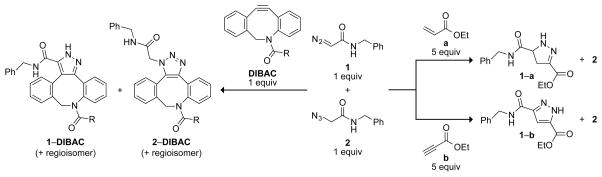
Scheme 1.
(Left) The 1,3-dipolar cycloaddition of a 1:1 mixture of diazoacetamide 1 and azidoacetamide 2 with a strained dipolarophile (R=CH2CH2NH2) was not selective. (Right) The 1,3-dipolar cycloaddition with unstrained dipolarophiles was selective for the diazo compound. Conditions: 1:1 CH3CN/H2O; ≤ 72 h; ambient temperature.