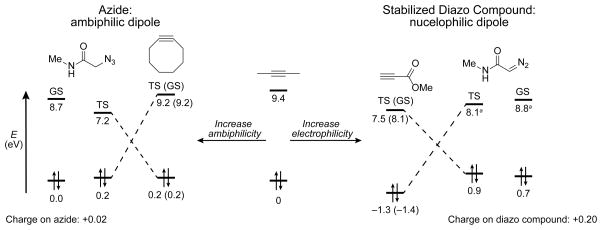
Figure 5.
Alternative principles govern the cycloadditions of azides and diazo compounds. Alkyne distortion increases ambiphilicity relative to 2-butyne, which is an optimal scenario for the ambiphilic azide dipole (left). Increasing electrophilicity primes the alkyne for increased reactivity with the more nucleophilic diazo compound (right). Orbital energies are given relative to the HOMO of 2-butyne for the ground state (GS) and transition state (TS) geometries, as indicated. aEnergies given for the LUMO+1 (see: Figures S3 and S5).